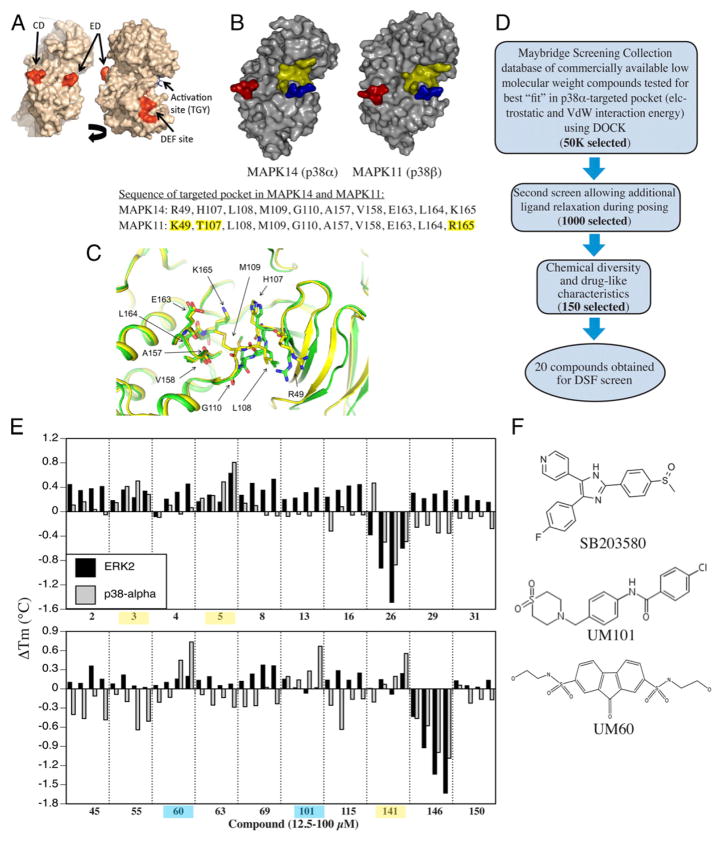
FIGURE 1.
Design of substrate-selective p38 inhibitors. (A) Structure of p38α showing CD, ED, DEF, and activation site. (B) Comparison of p38α and β structure. CD and ED sites colored red and blue and the CADD target yellow. The sequence comprising the CADD target on p38α and the corresponding site on p38β differ in only 3 of 10 aa (highlighted yellow). (C) Overlap of CADD target structure in apo- (PDB:1P38; green) and dual-phosphorylated (PDB:3PY3; yellow) mouse p38α. (D) Overview of CADD screening strategy. (E) DSF screening of compounds added at 10, 25, 50, or 100 μM to recombinant p38α or ERK2 with binding indicated by increase in melting temperature. Compounds binding ERK2 and p38 α highlighted yellow. Those only binding to p38 α are highlighted in blue. (F) Chemical structure of UM60, UM101, and SB203580.