Fig. 1. Evolutionary pressures on enzymes during adaptation to environmental temperature changes.
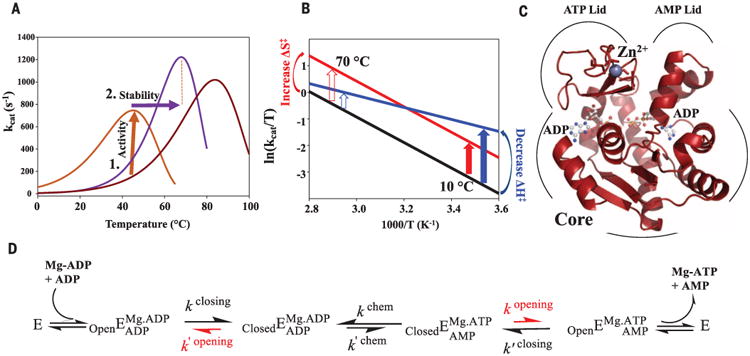
(A) Hypothetical ancestral protein (dark red) needs to increase activity at lower temperatures (brown arrow), resulting in a second ancestral enzyme (brown). Evolution from cold to hot imposes pressure on increased stability (purple arrow), resulting in a modern thermophile (purple). (B) Eyring plot illustrating proposed mechanisms for increasing enzymatic activity at colder temperatures by increasing ΔS‡ (red) versus decreasing ΔH‡ (blue) relative to the uncatalyzed reaction (black) (12, 13, 34) with larger rate acceleration at low temperatures by ΔH‡ (blue arrow). (C) The 1.2 Å x-ray structure of ANC1 Adk with two ADPs bound. (D) Reaction scheme for Adk catalysis, highlighting lid opening as the rate-limiting step (red) [from (22)].
