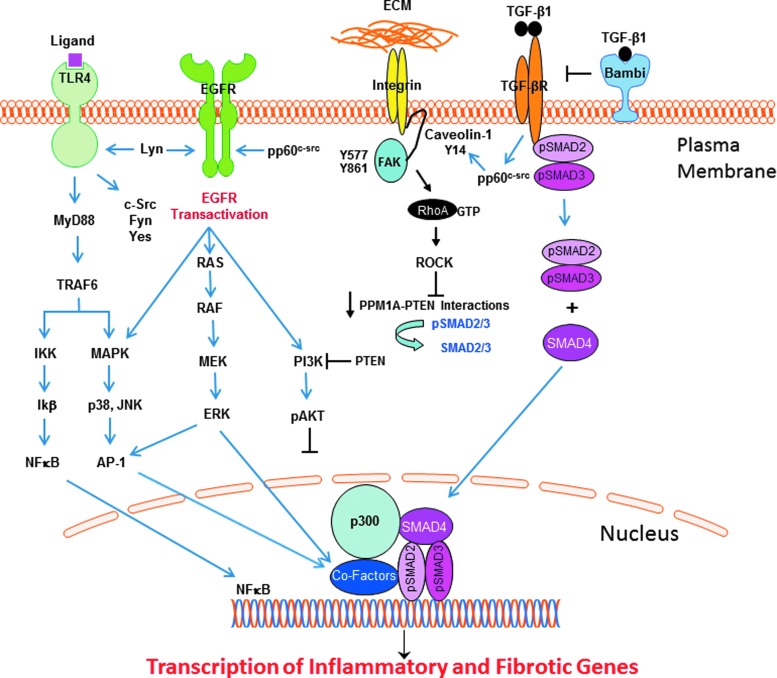
Figure 2.
TLR4 signaling in response to ligand binding, either directly to the TLR or through cooperative interactions with various coreceptors, mobilizes two major core parallel pathways, resulting in the activation of the transcription factor NF-κB and the p38/JNK MAP kinases via the upstream intermediate MyD88. The collateral or noncanonical pathways that impact TLR4 signaling outcomes involve several Src family kinases, transactivation of the EGFR (and downstream signaling intermediates), integrins, and the caveolin-1/FAK/RhoA/Rock/PTEN axis, as well as the canonical SMAD-dependent TGF-β pathway. The complex interactions among these diverse, although inter-related, pathways positively and negatively regulate the outcome of TLR4 signaling (detailed in this review). The available data suggest that while Akt activation may attenuate the amplitude and duration of the inflammatory response following TLR4 signaling, TGF-β1 regulation of RhoA/ROCK signaling controls PTEN activity that, in turn, affects SMAD2/3 transcription of the profibrotic genes (e.g., PAI-1, CTGF) through the SMAD phosphatase PPM1A. Akt, protein kinase B; CTGF, connective tissue growth factor; ECM, extracellular matrix; EGFR, epidermal growth factor receptor; PAI-1, plasminogen activator inhibitor-1; PTEN, phosphatase and tensin homolog; ROCK, rho-associated, coiled-coil-containing protein kinase; SMAD, Sma+mothers against decapentaplegic; TGF-β, transforming growth factor-β; TLR4, toll-like receptor 4.