Figure 1.
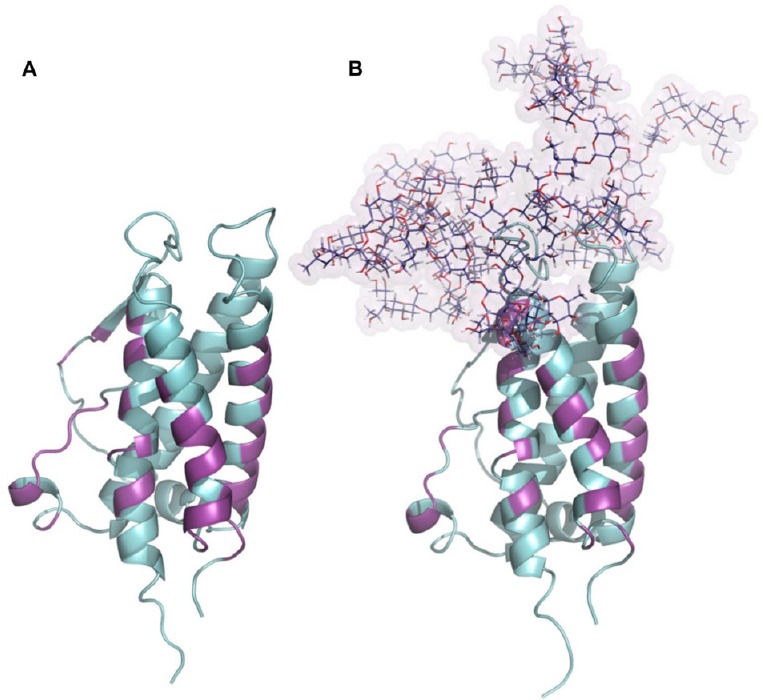
Glycosylation of erythropoietin.
(A) Structure of human erythropoietin obtained from the PDB: 1EER. (B) N-glycosylated model of erythropoietin generated combining each tetra-antennas glycan to their respective amino acids in erythropoietin sequence, N24, N38 and N83. The presence of N-glycosylations did not compromise the binding site to the erythropoietin receptor. All amino acids involved in erythropoietin-erythropoietin receptor interaction were colored magenta and define regions which remain largely conserved between both glycosylation states.
