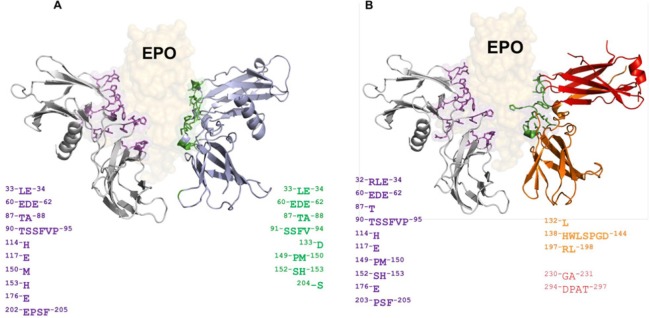
Figure 3.
In silico prediction of interaction between Epo and EpoR/EpoRβ.
(A) Complex Epo/EpoR generated by molecular docking where amino acids on the surface of EpoR involved in the interaction with Epo have been highlighted and colored according to their corresponding chain. For the classical EpoR, its functional conformation corresponds to a homodimer structure, and the two colors used (gray and light blue) for each chain differ only for schematic purposes. (B) Complex Epo/EpoRβ generated in a manner similar to the previous complex, with the exception that EpoRβ is a functional heterodimer, incorporating the beta common subunit formed by two interlaced chains colored red and orange, respectively. The number of amino acids in the interface is slightly lower than Epo/EpoR, however, both receptors are able to interact with Epo in a stable and energetically favorable manner. Epo: Erythropoietin; EpoR: Epo receptor.