Abstract
OBJECTIVE:
The aim of this meta-analysis was to evaluate the clinical efficacy of constraint-induced movement therapy in acute and sub-acute stroke.
DATA SOURCES:
The key words were stroke, cerebrovascular accident, constraint-induced therapy, forced use, and randomized controlled trial. The databases, including China National Knowledge Infrastructure, WanFang, Weipu Information Resources System, Chinese Biomedical Literature Database, PubMed, Medline, Embase, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews, were searched for studies on randomized controlled trials for treating acute or sub-acute stroke published before March 2016.
DATA SELECTION:
We retrieved relevant randomized controlled trials that compared constraint-induced movement therapy in treatment of acute or sub-acute stroke with traditional rehabilitation therapy (traditional occupational therapy). Patients were older than 18 years, had disease courses less than 6 months, and were evaluated with at least one upper extremity function scale. Study quality was evaluated, and data that met the criteria were extracted. Stata 11.0 software was used for the meta-analysis.
OUTCOME MEASURES:
Fugl-Meyer motor assessment of the arm, the action research-arm test, a motor activity log for amount of use and quality of movement, the Wolf motor function test, and a modified Barthel index.
RESULTS:
A total of 16 prospective randomized controlled trials (379 patients in the constraint-induced movement-therapy group and 359 in the control group) met inclusion criteria. Analysis showed significant mean differences in favor of constraint-induced movement therapy for the Fugl–Meyer motor assessment of the arm (weighted mean difference (WMD) = 10.822; 95% confidence intervals (95% CI): 7.419–14.226), the action research-arm test (WMD = 10.718; 95% CI: 5.704–15.733), the motor activity log for amount of use and quality of movement (WMD = 0.812; 95% CI: 0.331–1.293) and the modified Barthel index (WMD = 10.706; 95% CI: 4.417–16.966).
CONCLUSION:
Constraint-induced movement therapy may be more beneficial than traditional rehabilitation therapy for improving upper limb function after acute or sub-acute stroke.
Keywords: nerve regeneration, stroke, constraint-induced movement therapy, meta-analysis, upper extremity function, rehabilitation, intensity, neural regeneration
Introduction
Stroke is a leading cause of disability, primarily resulting from motor impairment (Dobkin, 2005; Towfighi and Saver, 2011; Corbetta et al., 2015). Although most patients show large improvement in motor function soon after stroke, 75% of patients continue to have upper extremity deficits 3–6 months later (Pang et al., 2006; Ng et al., 2007; Chen et al., 2017).
Constraint-induced movement therapy (CIMT) is a neurorehabilitatory approach developed by Taub et al. (1993) that is characterized by restraint of the less affected upper limb and forced use of the affected arm. This is usually achieved by placing the less affected arm in a padded mitten and then engaging in extensive task-oriented training of the affected arm for up to 90% of daily waking hours, 2 weeks per month (14 days in total) (Taub and Wolf, 1997; Kwakkel et al., 2015). However, receiving intensive occupational therapy for 6 hours every day leads to a low level of treatment compliance. To overcome this difficulty, a modified CIMT (mCIMT) has been developed in last few decades (Page et al., 2001, 2002; Wang et al., 2011; Taub et al., 2013; Souza et al., 2015; Zhu et al., 2016). The mCIMT is characterized by lower intensity training compared with traditional CIMT.
An increasing number of studies have demonstrated that CIMT after stroke, especially in the chronic phase (> 6 months), is more effective than standard rehabilitation measures (van der Lee et al., 1999; Wolf et al., 2006; Sterr et al., 2014; Park et al., 2015; Takebayashi et al., 2015; Ballester et al., 2016). However, whether CIMT has higher efficacy than conventional rehabilitation in acute or sub-acute stroke remains a key question. Some studies have shown that CIMT is not suitable for rehabilitation in patients with acute stroke. High-intensity CIMT started in the first days and weeks post stroke may aggravate limb function deterioration (Dromerick et al., 2009). Additionally, animal experiments have proved that immediate casting of the unaffected forelimb may cause lesion enlargement that is presumed excitotoxic and is associated with a decrement in motor recovery (Kozlowski et al., 1996; Humm et al., 1999; DeBow et al., 2004; Diederich et al., 2012). However, some studies have indicated that CIMT interventions during the acute phase have a positive effect on upper limb motor function (Thrane et al., 2015). Nevertheless, a systematic evaluation of these studies is required to accurately understand the efficacy of CIMT.
Some systematic reviews have focused on the effects of mCIMT on upper limb motor function in patients with chronic stroke (Bonaiuti et al., 2007; Shi et al., 2011; Peurala et al., 2012; Janssen et al., 2013). To the best of our knowledge, only one systematic review has focused on CIMT/mCIMT in acute or sub-acute stroke (Nijland et al., 2011). However, these results were based on only five studies. Even fewer articles were included in the calculation of a single index, which had an inevitable negative impact on the reliability of the results. Further studies with larger sample sizes have been conducted since 2011 when the original review was published; therefore, a new systematic review is essential.
The purpose of this meta-analysis was to explore the effects of mCIMT on upper extremity motor function in patients with acute or sub-acute stroke. The results are based on an evaluation of CIMT efficacy for arm motor function and assessments of behavioral techniques, hours of training, and the time from stroke occurrence to trial enrollment.
Methods
Literature and search strategy
Potentially relevant literature was identified through computerized and manual searches. A number of publications were searched using MeSH terms and free words. The databases from which articles were sourced included China National Knowledge Infrastructure, WanFang, Weipu Information Resources System, Chinese Biomedical Literature Database, PubMed, Medline, Embase, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews. The review period extended from the inception of each database to March 2016. The key words were stroke, cerebrovascular accident, constraint-induced therapy, forced use, and clinical trial. The language search was limited to English and Chinese. The following MeSH headings and key words were used: stroke, cerebrovascular accident, constraint-induced therapy, forced use, and randomized controlled trial. Additional relevant articles not captured by these databases were identified by reviewing references listed in the retrieved articles. This paper was prepared in accordance with the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines.
Study selection
Studies published before March 2016 were included if they met the following inclusion criteria: (1) a randomized clinical trial that included adult patients (≥ 18 years); (2) a CIMT/mCIMT group and a control group that received traditional rehabilitation therapy; (3) at least one of the following assessment methods: Fugl-Meyer motor assessment of the arm (FMA), the action research-arm test (ARAT), a motor activity log (MAL) for amount of use (AOU) and quality of movement (QOM), the Wolf motor function test (WMFT), or a modified Barthel index (mBI); (4) participants were in the acute or sub-acute phase after having a stroke (< 6 months); (5) published in English or Chinese.
Exclusion criteria: Patients meeting any of the following criteria were excluded (1) other rehabilitation therapies. This was to more specifically assess the specific effects of CIMT; (2) non-randomized trials.
Two authors (Xi-hua Liu and Juan Huai) assessed the identified articles by reading titles and abstracts, to confirm that they satisfied the inclusion criteria. When a study did not contain sufficient information in the abstract to make a decision, the full text was reviewed. In the event of disagreement, a third reviewer (Shou-wei Yue) was consulted.
Quality assessment
Study quality of each article was assessed using the Physiotherapy Evidence Database (PEDro) scale (Maher et al., 2003) independently from each other. PEDro is a valid scale consisting of 11 items. One point was given for each criterion that was satisfied, with a maximum score of 10. A study scoring 4 or higher was considered to be high quality (Maher et al., 2003; Van Peppen et al., 2004). In the event of disagreement, a third reviewer (Shou-wei Yue) was consulted.
Statistical analysis
For each outcome variable, the results were pooled by calculating the weighted mean difference (WMD) and 95% confidence intervals (95% CI) when the outcomes were reported on the same scale. The WMD and the corresponding standard deviation were calculated using the difference in the post-intervention means between the experimental and control groups. The Q test was administered to test for between-study homogeneity, which was set at a significance level of 10%. A random effects model was used to calculate the pooled outcome variables if significant heterogeneity was found (I2 ≥ 50%). For I2 < 50%, a fixed-effect model was used. Subgroup analyses were further conducted according to the degree of mCIMT, which deemed high-intensity (HI) or low-intensity (LO) according to the VECTORS study (Dromerick et al., 2009). Sensitivity analysis was also performed to assess the stability of the results. Publication bias was assessed using Begg's test and Egger's test, which were set at a significance level of 5%. Statistical analyses were conducted using STATA version 11 (StataCorp LP, College Station, TX, USA).
Results
Data retrieval
As shown in Figure 1, 1,086 potentially relevant studies were identified according to the literature search strategy. Of these, 623 were identified after removing duplicates. Subsequently, 518 studies were excluded based on the title and abstract. Of the remaining 105 studies, 89 were excluded after full-text review for varying reasons. For example, one paper was excluded because it did not provide sufficient data in the calculations (Brunner et al., 2012), while another was excluded because the patients in the control group received bimanual training rather than traditional rehabilitation therapy (Batool et al., 2015). Thus 16 prospective studies comprising 738 participants were included in the meta-analysis (Dromerick et al., 2000, 2009; Page et al., 2005; Ro et al., 2006; Boake et al., 2007; He et al., 2010; Wu et al., 2010; Zhang et al., 2011, 2015a; Singh and Pradhan, 2013; Huang et al., 2014; Yoon et al., 2014; El-Helow et al., 2015; Thrane et al., 2015; Liu et al., 2016; Song et al., 2016).
Figure 1.
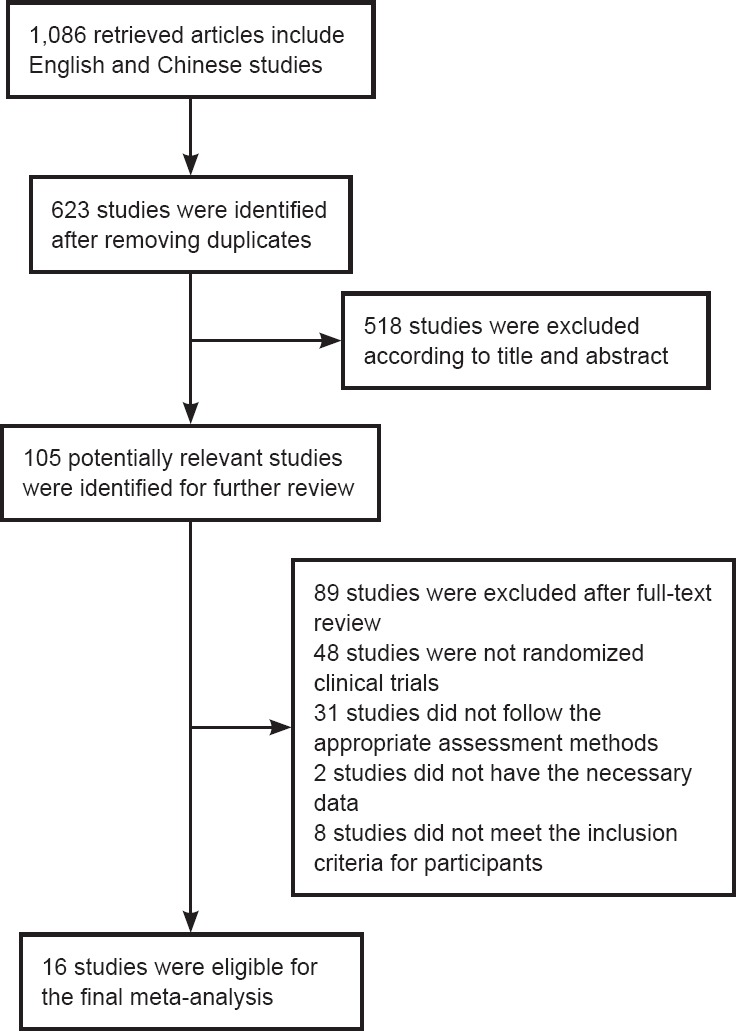
Flow chart of the exclusion/inclusion process.
Characteristics of the studies
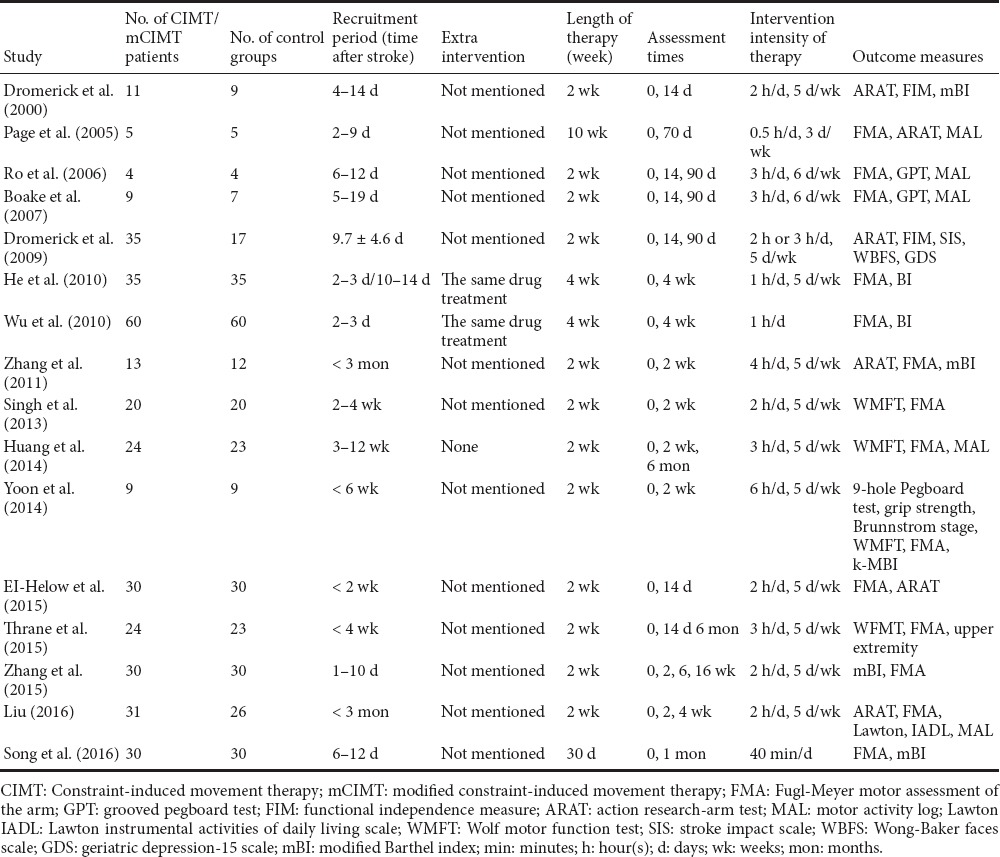
Of the 16 studies included in the final meta-analysis, 10 were written in English and 6 were in Chinese (Table 1). The effects of CIMT/mCIMT were evaluated in 5 studies using the ARAT, 6 using an mBI, 13 using the FMA, 4 using an MAL (AOU and QOU), and 2 using the WMFT.
Table 1.
Characteristics of the 16 included studies
Quality assessment
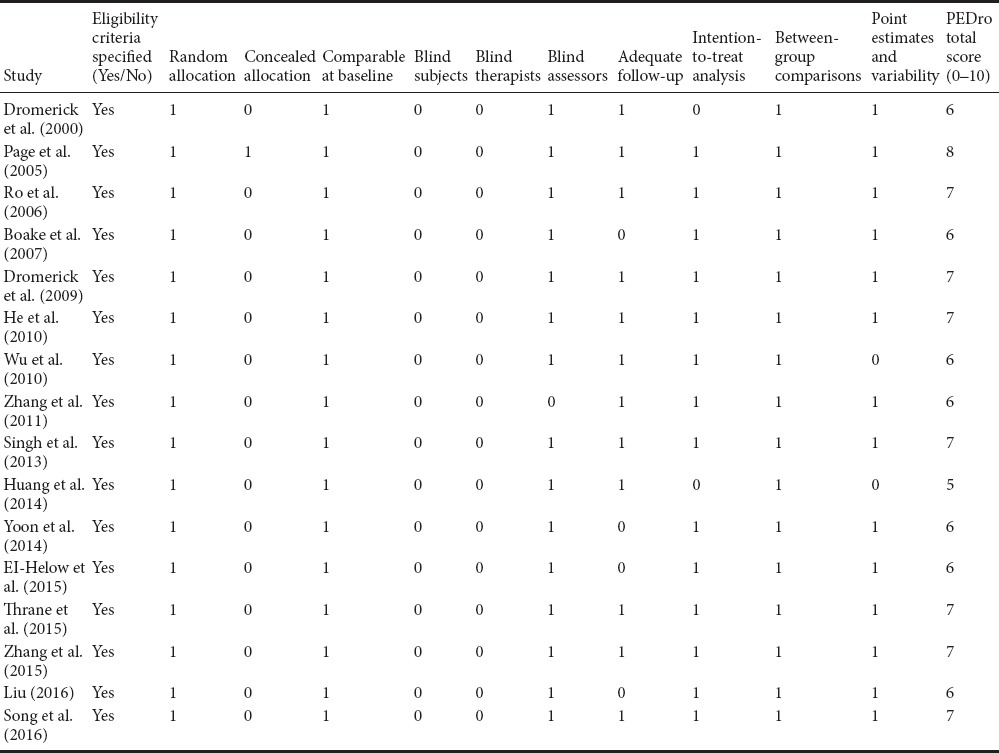
Table 2 shows the quality assessment scores for the included studies, according to the PEDro scale. The PEDro scores ranged from 5 to 8 points, with a median score of 6.5 points. No study was excluded from further analysis.
Table 2.
Methodological quality of the included studies - assessed with the 10-item Physiotherapy Evidence Database (PEDro) scale
Meta-analysis results
ARAT
Five studies (Dromerick et al., 2000, 2009; Page et al., 2005; Zhang et al., 2011; El-Helow et al., 2015) assessed CIMT/mCIMT efficacy using the ARAT. Figure 2A and Table 3 show a significantly heterogeneous WMD (WMD [random]: 8.35; 95% CI: 1.98–14.71; Z = 4.19; P = 0.001; I2 = 94.1%).
Figure 2.
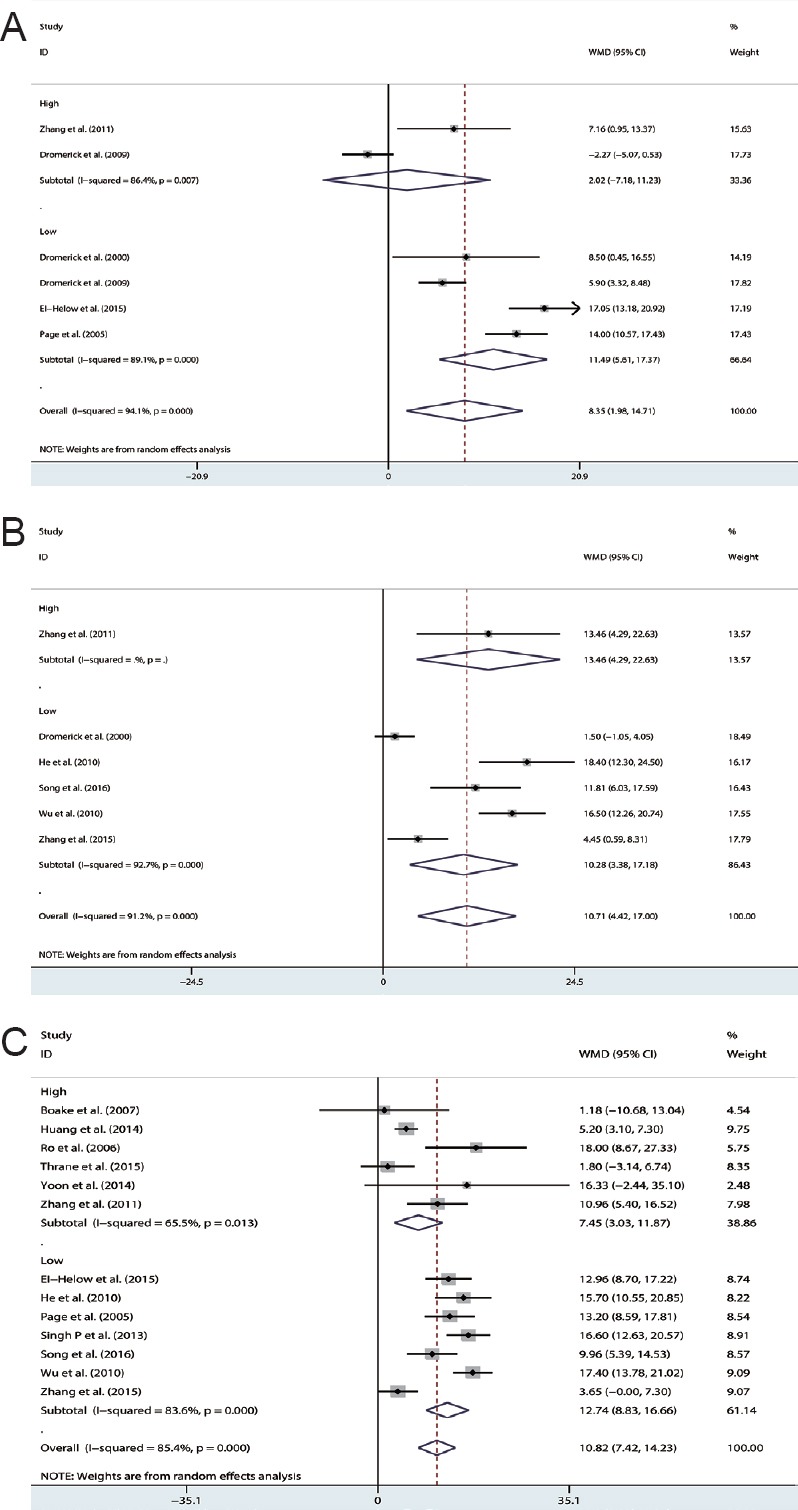
Effects of CIMT on the arm motor function of patients with acute or sub-acute stroke. Assessments included the ARAT (A), mBI (B), and FMA (C).
CIMT: Constraint-induced movement therapy; ARAT: action research-arm test; mBI: modified Barthel index; FMA: Fugl–Meyer motor assessment of the arm.
Table 3.
Meta-analysis of constraint-induced movement therapy in acute and sub-acute stroke
The two studies (Dromerick et al., 2009; Zhang et al., 2011) that used HI CIMT yielded a nonsignificant difference in favor of the control group (WMD [random]: 2.02; 95% CI: –7.18–11.23; P = 0.667). The four studies (Dromerick et al., 2000; Page et al., 2005; Dromerick et al., 2009; El-Helow et al., 2015) that used LO CIMT yielded a significant WMD in favor of the experimental group (WMD: 11.49; 95% CI: 5.61–17.37; P < 0.001).
mBI
Six studies (Dromerick et al., 2000; He et al., 2010; Wu et al., 2010; Zhang et al., 2011, 2015b; Song et al., 2016) assessed the efficacy of CIMT/mCIMT on basic activities of daily living function using an mBI. Figure 2B and Table 3 show a significantly heterogeneous total standardized mean difference (SMD) for the mBI (SMD [random]:10.706; 95% CI: 4.417–16.966; Z = 3.34; P = 0.001; I2 = 91.2%).
One study used HI CIMT (SMD [random]: 13.46; 95% CI: 4.29–22.63; Z = 2.88; P = 0.004) and four studies used LO CIMT (SMD [random]: 10.28; 95% CI: 3.38–17.18; Z = 2.92; P = 0.003). Both HI and LO CIMT yielded significantly better mBI values than the control group.
FMA
Thirteen studies (Page et al., 2005; Ro et al., 2006; Boake et al., 2007; He et al., 2010; Wu et al., 2010; Zhang et al., 2011, 2015b; Singh and Pradhan, 2013; Huang et al., 2014; Yoon et al., 2014; El-Helow et al., 2015; Thrane et al., 2015; Song et al., 2016) were evaluated to determine the effects of CIMT/mCIMT on motor impairment using the FMA. Figure 2C and Table 3 show a significantly heterogeneous total SMD for the FMA (WMD [random] = 10.822; 95% CI: 7.419–14.226; Z = 6.23; P < 0.001; I2 = 85.4%; Figure 2C).
The studies that used HI CIMT and LO CIMT yielded significant differences. The subtotal studies that used HI CIMT and LO CIMT also yielded significant differences. The subtotal WMD for HI CIMT studies (WMD [random] = 7.45; 95% CI: 3.03–11.87; Z = 3.31; P = 0.001; I2 = 65.5%) was lower than the subtotal WMD for LO CIMT studies (WMD[random] = 12.74; 95% CI: 8.83–16.66; Z = 6.23; P < 0.001; I2 = 83.6%; Figure 2C).
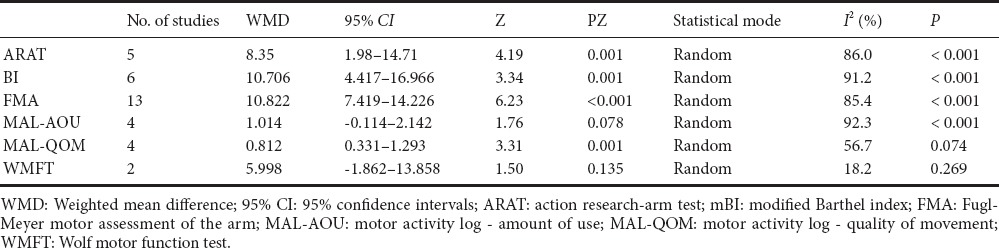
MAL
Four studies (Page et al., 2005; Ro et al., 2006; Boake et al., 2007; Huang et al., 2014) assessed the efficacy of CIMT/mCIMT using an MAL. Figure 3 shows that the meta-analysis results yielded a non-significant heterogeneous WMD for AOU (WMD [random] = 1.014; 95% CI: (–0.114, 2.142); Z = 1.76; P = 0.078; I2 = 92.3%), but a significant difference for QOU (WMD [random] = 0.812; 95% CI: 0.331–1.293; Z = 3.31; P = 0.001; I2 = 56.7%).
Figure 3.
Effects of CIMT on arm motor function of patients with acute or sub-acute. Assessments include the AOU (A), QOM (B), and WMFT (C).
CIMT: Constraint-induced movement therapy; AOU: amount of use; QOM: quality of movement; WMFT: Wolf motor function test; WMD: weighted mean difference; 95% CI: 95% confidence intervals.
The studies using HI or LO CIMT yielded significant differences in favor of the experimental group for AOU (WMDHI [random] = 0.61, 95% CI: 0.20–1.01, Z = 2.94, P = 0.003; WMDLO [random] = 2.18; 95% CI: 1.89–2.47; Z = 3.31; P = 0.001) and QOU (WMDHI [random] = 0.55, 95% CI: 0.14–0.97, Z = 2.64, P = 0.008; WMDLO [random] = 1.21; 95% CI: 0.93–1.49; Z = 8.38; P < 0.001).
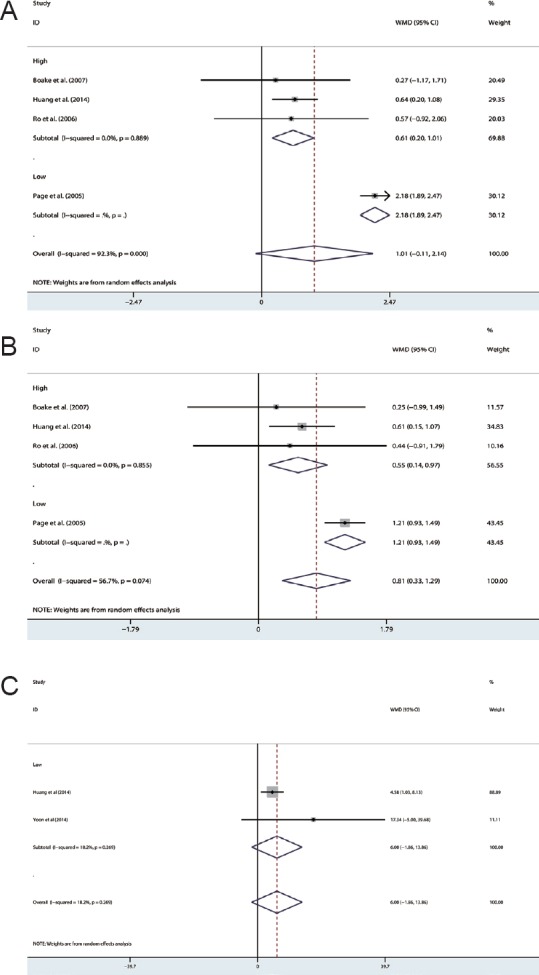
WMFT
Two studies (Huang et al., 2014; Yoon et al., 2014) assessed the efficacy of CIMT/mCIMT using the WMFT. Pooling the results indicated a non-significant heterogeneous WMD (WMD [fixed] = 5.998; 95% CI: –1.862–13.858; Z = 1.50; P = 0.135; I2 = 18.2%; Figure 3C and Table 3).
Publication bias and sensitivity analyses
As shown in Table 4, all P-values from the Begg's test (0.34–1.00) and the Egger's test (0.075–0.488) were greater than 0.05, indicating that no publication bias was detected. Results of the sensitivity analyses were similar to those of the overall meta-analysis (P > 0.05).
Table 4.
Publication bias assessed by Begg's test and Egger's test (P)

Discussion
Analysis of efficacy
To the best of our knowledge, the present study represents the second systematic review to quantitatively investigate the clinical efficacy of CMIT in acute or sub-acute stroke. Compared with the first systematic review, we included more studies with larger sample sizes, which should strengthen the reliability of our conclusions. We found that CIMT or mCIMT may be more beneficial in acute or sub-acute stroke than traditional rehabilitation therapy. A total of 379 CIMT patients and 359 healthy controls were included in the meta-analysis, which greatly improved the statistical power and credibility of the conclusions over what was possible considering each study individually. The times for clinical outcome assessment differed among the studies. In our meta-analysis, we only compared the clinical outcomes after rehabilitation therapy to reduce any heterogeneity among the studies.
CIMT/mCIMT had significant effects on arm motor function and activities of daily living in acute and sub-acute stroke. However, no significant difference was found on the motor activity log-amount of use (MAL-AOU) or the WMFT. The results of subgroup analysis by CIMT degree also yielded significantly positive WMDs for most outcome measures compared with the control group. The I2 values in both subgroups became smaller compared with the total value, which indicated that heterogeneity decreased among the included studies. This might suggest that the amount of CIMT training was one critical factor that affected the clinical results. Critically, the WMDs for LO CIMT were larger than those for HI CIMT, which suggests that LO CIMT may be better than HI CIMT in acute and sub-acute stroke.
The biological mechanism underlying the efficacy of CIMT in acute and sub-acute stroke is still unclear (Zhao et al., 2009, 2013; Zhang et al., 2015b). Experimental studies have shown that hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke (Cook et al., 2017). Our analysis showed that the results from the included studies differed from each other in some aspects. The main reason is likely the variable inclusion criteria. Patients in all studies were recruited at the acute or sub-acute phase (< 4 months) after stroke; however, this is still a relatively long period. The recruitment period in most of the studies was set at less than 2 weeks, while the recruitment period in the studies by Singh and Pradhan (2013) and Yoon et al. (2014) were greater than 2 weeks. Such differences in the inclusion criteria might have a substantial influence on the clinical effects. Another reason for the heterogeneity might be the variations in the intensity and duration of the intervention. For instance, the patients in most of the studies received 2 weeks of therapy for 2 or 3 hours per day, while in some studies, the patients received longer (4 or 10 weeks) or more intensive (4 or 6 hours per day) interventions. Inevitably, this also had a strong influence on the clinical effects.
Potential biases in the present study
Begg's test and Egger's test were used to explore the potential publication bias. Although no publication bias was detected, publication bias should also not be ignored because null results tend not to be published. Studies with large positive results are often much easier to publish than studies with negative results (Papageorgiou et al., 2015; Sedgwick, 2015). Therefore, we cannot rule out publication bias.
Limitations of the present meta-analysis
The present study has several limitations. First, we only compared the short-term efficacies of the interventions for the two groups. The long-term efficacy (more than 3 months) was not included because the data were limited, although its clinical significance holds greater value. Second, there was heterogeneity of results among the included studies even though the inclusion criteria were clearly defined in the present study. The recruitment period (days after stroke) and intensity of therapy differed across the studies, and this inevitably affected the reliability of the meta-analysis results.
Conclusion
In summary, the present meta-analysis demonstrated that CIMT or mCIMT might be more beneficial than traditional rehabilitation therapy in the acute and sub-acute stroke. Furthermore, LO CIMT may be better than HI CIMT. These findings might have clinical significance for the rehabilitation of patients within acute or sub-acute stroke. However, large-scale, well-designed multi-center studies are needed to further confirm the impact that degree of CIMT or mCIMT has on functional outcomes in acute and sub-acute stroke.
Acknowledgments
The authors would like to thank Kewei Yue from Jiangnan University of China for his cooperation in the statistical analysis.
Footnotes
Funding: This study was supported by the Natural Science Foundation of Shandong Province of China, No. 2014ZRB14502.
Conflicts of interest: None declared.
Data sharing statement: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
References
- Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- Ballester BR, Maier M, San Segundo Mozo RM, Castaneda V, Duff A, M J Verschure PF. Counteracting learned non-use in chronic stroke patients with reinforcement-induced movement therapy. J Neuroeng Rehabil. 2016;13:74. doi: 10.1186/s12984-016-0178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batool S, Soomro N, Amjad F, Fauz R. To compare the effectiveness of constraint induced movement therapy versus motor relearning programme to improve motor function of hemiplegic upper extremity after stroke. Pak J Med Sci. 2015;31:1167–1171. doi: 10.12669/pjms.315.7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boake C, Noser EA, Ro T, Baraniuk S, Gaber M, Johnson R, Salmeron ET, Tran TM, Lai JM, Taub E, Moye LA, Grotta JC, Levin HS. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil Neural Repair. 2007;21:14–24. doi: 10.1177/1545968306291858. [DOI] [PubMed] [Google Scholar]
- Bonaiuti D, Rebasti L, Sioli P. The constraint induced movement therapy: a systematic review of randomised controlled trials on the adult stroke patients. Eura Medicophys. 2007;43:139–146. [PubMed] [Google Scholar]
- Brunner IC, Skouen JS, Strand LI. Is modified constraint-induced movement therapy more effective than bimanual training in improving arm motor function in the subacute phase post stroke? A randomized controlled trial. Clin Rehabil. 2012;26:1078–1086. doi: 10.1177/0269215512443138. [DOI] [PubMed] [Google Scholar]
- Chen CM, Yang YH, Chang CH, Chen PC. Effects of transferring to the rehabilitation ward on long-term mortality rate of first-time stroke survivors: a population-based study. Arch Phys Med Rehabil. 2017 doi: 10.1016/j.apmr.2017.03.020. doi: 10.1016/j.apmr.2017.03.020. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Nguyen C, Chun HN, I LL, Chiu AS, Machnicki M, Zarembinski TI, Carmichael ST. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J Cereb Blood Flow Metab. 2017;37:1030–1045. doi: 10.1177/0271678X16649964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta D, Sirtori V, Castellini G, Moja L, Gatti R. Constraint-induced movement therapy for upper extremities in people with stroke. Cochrane Database Syst Rev. 2015:Cd004433. doi: 10.1002/14651858.CD004433.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBow SB, McKenna JE, Kolb B, Colbourne F. Immediate constraint-induced movement therapy causes local hyperthermia that exacerbates cerebral cortical injury in rats. Can J Physiol Pharmacol. 2004;82:231–237. doi: 10.1139/y04-013. [DOI] [PubMed] [Google Scholar]
- Diederich K, Quennet V, Bauer H, Muller HD, Wersching H, Schabitz WR, Minnerup J, Sommer C. Successful regeneration after experimental stroke by granulocyte-colony stimulating factor is not further enhanced by constraint-induced movement therapy either in concurrent or in sequential combination therapy. Stroke. 2012;43:185–192. doi: 10.1161/STROKEAHA.111.622159. [DOI] [PubMed] [Google Scholar]
- Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med. 2005;352:1677–1684. doi: 10.1056/NEJMcp043511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromerick AW, Edwards DF, Hahn M. Does the application of constraint-induced movement therapy during acute rehabilitation reduce arm impairment after ischemic stroke? Stroke. 2000;31:2984–2988. doi: 10.1161/01.str.31.12.2984. [DOI] [PubMed] [Google Scholar]
- Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, Powers WJ, Wolf SL, Edwards DF. Very early constraint-induced movement during stroke rehabilitation (VECTORS): A single-center RCT. Neurology. 2009;73:195–201. doi: 10.1212/WNL.0b013e3181ab2b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Helow MR, Zamzam ML, Fathalla MM, El-Badawy MA, El Nahhas N, El-Nabil LM, Awad MR, Von Wild K. Efficacy of modified constraint-induced movement therapy in acute stroke. Eur J Phys Rehabil Med. 2015;51:371–379. [PubMed] [Google Scholar]
- He ZY, Tu XH, Fu X. Clinical study on constraint-induced movement in acute stroke with upper limb disorder. Linchuang Heli Yongyao Zazhi. 2010;3:24–25. [Google Scholar]
- Huang HH, Wang LX, Zhang QX, Wu LF, Lin RT. The effects of constraint-induced movement therapy on early stage upper limb function recovery in patients with subacute stroke. Zhonghua Wuli Yixue yu Kangfu Zazhi. 2014;36:838–841. [Google Scholar]
- Humm JL, Kozlowski DA, Bland ST, James DC, Schallert T. Use-dependent exaggeration of brain injury: is glutamate involved? Exp Neurol. 1999;157:349–358. doi: 10.1006/exnr.1999.7061. [DOI] [PubMed] [Google Scholar]
- Janssen H, Speare S, Spratt NJ, Sena ES, Ada L, Hannan AJ, McElduff P, Bernhardt J. Exploring the efficacy of constraint in animal models of stroke: meta-analysis and systematic review of the current evidence. Neurorehabil Neural Repair. 2013;27:3–12. doi: 10.1177/1545968312449696. [DOI] [PubMed] [Google Scholar]
- Kozlowski DA, James DC, Schallert T. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci. 1996;16:4776–4786. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakkel G, Veerbeek JM, van Wegen EE, Wolf SL. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015;14:224–234. doi: 10.1016/S1474-4422(14)70160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KP, Balderi K, Leung TL, Yue AS, Lam NC, Cheung JT, Fong SS, Sum CM, Bissett M, Rye R, Mok VC. A randomized controlled trial of self-regulated modified constraint-induced movement therapy in sub-acute stroke patients. Eur J Neurol. 2016;23:1351–1360. doi: 10.1111/ene.13037. [DOI] [PubMed] [Google Scholar]
- Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- Ng YS, Stein J, Ning M, Black-Schaffer RM. Comparison of clinical characteristics and functional outcomes of ischemic stroke in different vascular territories. Stroke. 2007;38:2309–2314. doi: 10.1161/STROKEAHA.106.475483. [DOI] [PubMed] [Google Scholar]
- Nijland R, Kwakkel G, Bakers J, van Wegen E. Constraint-induced movement therapy for the upper paretic limb in acute or sub-acute stroke: a systematic review. Int J Stroke. 2011;6:425–433. doi: 10.1111/j.1747-4949.2011.00646.x. [DOI] [PubMed] [Google Scholar]
- Page SJ, Levine P, Leonard AC. Modified constraint-induced therapy in acute stroke: a randomized controlled pilot study. Neurorehabil Neural Repair. 2005;19:27–32. doi: 10.1177/1545968304272701. [DOI] [PubMed] [Google Scholar]
- Page SJ, Sisto SA, Levine P, Johnston MV, Hughes M. Modified constraint induced therapy: a randomized feasibility and efficacy study. J Rehabil Res Dev. 2001;38:583–590. [PubMed] [Google Scholar]
- Page SJ, Sisto S, Johnston MV, Levine P, Hughes M. Modified constraint-induced therapy in subacute stroke: a case report. Arch Phys Med Rehabil. 2002;83:286–290. doi: 10.1053/apmr.2002.28007. [DOI] [PubMed] [Google Scholar]
- Pang MY, Harris JE, Eng JJ. A community-based upper-extremity group exercise program improves motor function and performance of functional activities in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2006;87:1–9. doi: 10.1016/j.apmr.2005.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou SN, Dimitraki D, Coolidge T, Kotsanos N. Publication bias & small-study effects in pediatric dentistry meta-analyses. J Evid Based Dent Pract. 2015;15:8–24. doi: 10.1016/j.jebdp.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Park J, Lee N, Cho Y, Yang Y. Modified constraint-induced movement therapy for clients with chronic stroke: interrupted time series (ITS) design. J Phys Ther Sci. 2015;27:963–966. doi: 10.1589/jpts.27.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peurala SH, Kantanen MP, Sjogren T, Paltamaa J, Karhula M, Heinonen A. Effectiveness of constraint-induced movement therapy on activity and participation after stroke: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2012;26:209–223. doi: 10.1177/0269215511420306. [DOI] [PubMed] [Google Scholar]
- Ro T, Noser E, Boake C, Johnson R, Gaber M, Speroni A, Bernstein M, De Joya A, Scott Burgin W, Zhang L, Taub E, Grotta JC, Levin HS. Functional reorganization and recovery after constraint-induced movement therapy in subacute stroke: case reports. Neurocase. 2006;12:50–60. doi: 10.1080/13554790500493415. [DOI] [PubMed] [Google Scholar]
- Sedgwick P. What is publication bias in a meta-analysis? BMJ. 2015;351:h4419. doi: 10.1136/bmj.h4419. [DOI] [PubMed] [Google Scholar]
- Shi YX, Tian JH, Yang KH, Zhao Y. Modified constraint-induced movement therapy versus traditional rehabilitation in patients with upper-extremity dysfunction after stroke: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2011;92:972–982. doi: 10.1016/j.apmr.2010.12.036. [DOI] [PubMed] [Google Scholar]
- Singh P, Pradhan B. Study to assess the effectiveness of modified constraint-induced movement therapy in stroke subjects: A randomized controlled trial. Ann Indian Acad Neurol. 2013;16:180–184. doi: 10.4103/0972-2327.112461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CG, Zhang RG, Zhang J, Liu JF, Yang H. The rehabilitation effect of constraint induced movement therapy on hemiplegic upper limb dysfunction in subacute period. Zhongguo Yiyao Kexue. 2016;6:149–151. [Google Scholar]
- Souza WC, Conforto AB, Orsini M, Stern A, Andre C. Similar effects of two modified constraint-induced therapy protocols on motor impairment, motor function and quality of life in patients with chronic stroke. Neurol Int. 2015;7:5430. doi: 10.4081/ni.2015.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterr A, O’Neill D, Dean PJ, Herron KA. CI therapy is beneficial to patients with chronic low-functioning hemiparesis after stroke. Front Neurol. 2014;5:204. doi: 10.3389/fneur.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi T, Amano S, Hanada K, Umeji A, Takahashi K, Marumoto K, Kodama N, Koyama T, Domen K. A one-year follow-up after modified constraint-induced movement therapy for chronic stroke patients with paretic arm: a prospective case series study. Top Stroke Rehabil. 2015;22:18–25. doi: 10.1179/1074935714Z.0000000028. [DOI] [PubMed] [Google Scholar]
- Taub E, Wolf SL. Constraint induced movement techniques to facilitate upper extremity use in stroke patients. Top Stroke Rehabil. 1997;3:38–61. doi: 10.1080/10749357.1997.11754128. [DOI] [PubMed] [Google Scholar]
- Taub E, Miller NE, Novack TA, Cook EW, 3rd, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- Taub E, Uswatte G, Mark VW, Morris DM, Barman J, Bowman MH, Bryson C, Delgado A, Bishop-McKay S. Method for enhancing real-world use of a more affected arm in chronic stroke: transfer package of constraint-induced movement therapy. Stroke. 2013;44:1383–1388. doi: 10.1161/STROKEAHA.111.000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrane G, Askim T, Stock R, Indredavik B, Gjone R, Erichsen A, Anke A. Efficacy of constraint-induced movement therapy in early stroke rehabilitation: a randomized controlled multisite trial. Neurorehabil Neural Repair. 2015;29:517–525. doi: 10.1177/1545968314558599. [DOI] [PubMed] [Google Scholar]
- Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42:2351–2355. doi: 10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Deville WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke. 1999;30:2369–2375. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- Van Peppen RP, Kwakkel G, Wood-Dauphinee S, Hendriks HJ, Van der Wees PJ, Dekker J. The impact of physical therapy on functional outcomes after stroke: what's the evidence? Clin Rehabil. 2004;18:833–862. doi: 10.1191/0269215504cr843oa. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhao JL, Zhu QX, Li J, Meng PP. Comparison of conventional therapy, intensive therapy and modified constraint-induced movement therapy to improve upper extremity function after stroke. J Rehabil Med. 2011;43:619–625. doi: 10.2340/16501977-0819. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- Wu DH, Liu XJ, Chen JH, Wu LX, Xiong J, He YY, Wang MS, Jiang XP. Clinical study of induced movement therapy in acute stroke patients on upper extremity function. The Proceedings of the 8th Annual Symposium on Neurology of Guizhou Medical Association. 2010:41–43. [Google Scholar]
- Yoon JA, Koo BI, Shin MJ, Shin YB, Ko HY, Shin YI. Effect of constraint-induced movement therapy and mirror therapy for patients with subacute stroke. Ann Rehabil Med. 2014;38:458–466. doi: 10.5535/arm.2014.38.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, He Q, Li YY, Li C, Bai YL. Constraint-induced movement therapy promotes motor function recovery and downregulates phosphorylated extracellular regulated protein kinase expression in ischemic brain tissue of rats. Neural Regen Res. 2015a;10:2004–2010. doi: 10.4103/1673-5374.172319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WM, Yang S, Wang YJ, He XY, Lu JC, Xie Q. Effect of modified constraint-induced movement therapy on the activities of daily living of patients with acute stroke. Zhongguo Xiandai Shenjing Jibing Zazhi. 2015b;15:280–284. [Google Scholar]
- Zhang ZC, Yang WT, Liao WJ, Liu Q. Impacts of the modified constraint-induced movement therapy on the upper limb function in acute cerebral apoplexy. Shijie Zhongxiyi Jiehe Zazhi. 2011;6:41–44. [Google Scholar]
- Zhao C, Wang J, Zhao S, Nie Y. Constraint-induced movement therapy enhanced neurogenesis and behavioral recovery after stroke in adult rats. Tohoku J Exp Med. 2009;218:301–308. doi: 10.1620/tjem.218.301. [DOI] [PubMed] [Google Scholar]
- Zhao S, Zhao M, Xiao T, Jolkkonen J, Zhao C. Constraint-induced movement therapy overcomes the intrinsic axonal growth-inhibitory signals in stroke rats. Stroke. 2013;44:1698–1705. doi: 10.1161/STROKEAHA.111.000361. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhou C, Liu Y, Liu J, Jin J, Zhang S, Bai Y, Huang D, Zhu B, Xu Y, Wu Y. Effects of modified constraint-induced movement therapy on the lower extremities in patients with stroke: a pilot study. Disabil Rehabil. 2016;38:1893–1899. doi: 10.3109/09638288.2015.1107775. [DOI] [PubMed] [Google Scholar]


