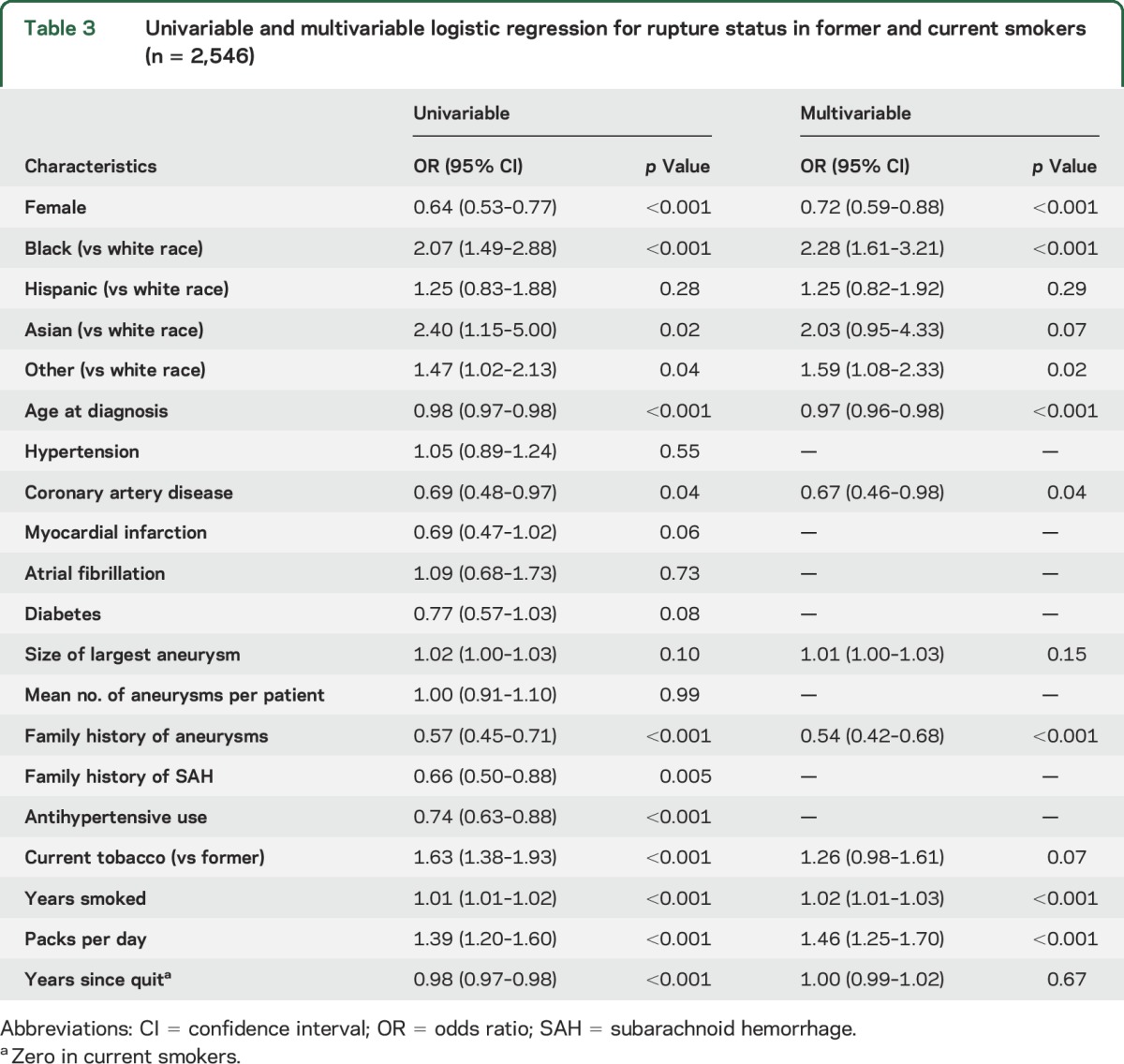
Abstract
Objective:
Although smoking is a known risk factor for intracranial aneurysm (IA) rupture, the exact relationship between IA rupture and smoking intensity and duration, as well as duration of smoking cessation, remains unknown.
Methods:
In this case-control study, we analyzed 4,701 patients with 6,411 IAs diagnosed at the Brigham and Women's Hospital and Massachusetts General Hospital between 1990 and 2016. We divided individuals into patients with ruptured aneurysms and controls with unruptured aneurysms. We performed univariable and multivariable logistic regression analyses to determine the association between smoking status and ruptured IAs at presentation. In a subgroup analysis among former and current smokers, we assessed the association between ruptured aneurysms and number of packs per day, duration of smoking, and duration since smoking cessation.
Results:
In multivariable analysis, current (odds ratio [OR] 2.21, 95% confidence interval [CI] 1.89–2.59) and former smoking status (OR 1.56, 95% CI 1.31–1.86) were associated with rupture status at presentation compared with never smokers. In a subgroup analysis among current and former smokers, years smoked (OR 1.02, 95% CI 1.01–1.03) and packs per day (OR 1.46, 95% CI 1.25–1.70) were significantly associated with ruptured aneurysms at presentation, whereas duration since cessation among former smokers was not significant (OR 1.00, 95% CI 0.99–1.02).
Conclusions:
Current cigarette smoking, smoking intensity, and smoking duration are significantly associated with ruptured IAs at presentation. However, the significantly increased risk persists after smoking cessation, and smoking cessation does not confer a reduced risk of aneurysmal subarachnoid hemorrhage beyond that of reducing the cumulative dose.
With an overall mortality rate of 45% and significant morbidity among survivors, aneurysmal subarachnoid hemorrhage (aSAH) places a disproportionate burden on our society.1 Although the exact underlying etiology that causes intracranial aneurysm (IA) rupture is not clearly understood, cigarette smoking is considered to be the most significant modifiable risk factor.2 The prevalence of smoking in patients with aSAH ranges from 45% to 75%, compared to 20%–35% in the general adult population.1–3 Despite the reported strength of this association in a variety of longitudinal4–14 and case-control studies,2,4,7,15–31 larger scale studies with a greater number of confirmed aSAH cases reporting detailed data regarding the effect of smoking status, dose rate, smoking duration, and duration of smoking cessation on the risk of aneurysm rupture are needed. Detailed knowledge regarding the risks of nicotine exposure on IA rupture may have important implications for screening and counseling of patients with IAs, and may lead to further hypotheses about the biological mechanisms by which smoking leads to vascular inflammation, hemodynamic stress, endothelial dysfunction, and ultimately, wall weakening and rupture. Most studies to date are based on a small number of patients with aSAH, ranging from 2621 to 426,16 variable diagnostic criteria, inclusion of other types of hemorrhagic stroke,6,7,9,29 restriction to a particular subgroup of patients according to age,30 sex,6,7,9 or profession,9 and control groups consisting of healthy participants instead of patients with unruptured aneurysms.2,15–19,21,22 Here we present one of the largest partially prospective single-institution series to date, to more precisely quantify the effect of smoking intensity, cumulative dose, and smoking cessation on rupture risk of IAs in 1,446 patients with ruptured and 4,045 patients with unruptured aneurysms.
METHODS
We evaluated the medical records of 4,701 patients who were diagnosed with an IA between 1990 and 2016 at the Brigham and Women's Hospital (BWH) and Massachusetts General Hospital (MGH). Patients were identified prospectively on clinical presentation (2007–2016) and retrospectively using natural language processing (NLP) in conjunction with the Partners Healthcare Research Patients Data Registry (RPDR), which includes 4.2 million patients who have received care from BWH and MGH (1990–2013).32 Briefly, we used ICD-9 and Current Procedural Terminology codes to obtain an initial set of potential aneurysm patients from the RPDR. We then used NLP to train a classification algorithm, which yielded 5,589 patients. There were also 1,139 patients who were obtained prospectively on clinical presentation, of whom 474 patients were seen from 2013 to 2016 and were in addition to the 5,589 patients obtained from NLP. The medical records of the 6,063 patients were reviewed in detail (A.C. and R. Du) to identify 4,701 patients with definite saccular aneurysms. The results of the imaging studies, including IA site and size, were recorded. The imaging studies of all patients with aneurysms ≤3 mm were reviewed and those with possible infundibula or nondefinitive aneurysms were excluded. We also excluded patients with feeding artery aneurysms associated with arteriovenous malformations, those lacking clinical notes or radiographic images, and those with fusiform or dissecting aneurysms from the present study. Patients whose aneurysms had been treated prior to presentation at our hospitals were also excluded from this study. We categorized patients who presented with aSAH as having a ruptured aneurysm.
Using patient notes and radiographic images, we compared the differences between ruptured and unruptured aneurysms in terms of the following: patient demographics (age, sex, race), comorbidities (hypertension, coronary artery disease, myocardial infarction, atrial fibrillation, diabetes mellitus), aneurysm characteristics (number of aneurysms, size of largest aneurysm), antihypertensive medication use, family history of IAs and aSAH, smoking history details (current smoker, former smoker, never smoker, number of packs per day, duration of smoking in years, and duration since cessation in former smokers). We categorized patients who quit smoking at least 1 year before diagnosis as former smokers. The presence of aSAH was confirmed with a CT scan, CSF analysis, or intraoperatively by a neurosurgeon. A comorbidity was assumed absent if we found no documentation of its presence. We obtained notes with smoking details by using the following search terms: smoke, smoked, smokes, smoker, smoking, ppd, pack years, py, packs per day, tobacco, cigarette, and cigarettes. We then obtained detailed smoking data manually.
Baseline characteristics were analyzed using the χ2 test for categorical variables and the t test for continuous variables. Categorical variables were reported as proportion and continuous variables were reported as mean (± SD). Each patient- and aneurysm-related variable was screened by univariable logistic regression for association with presence or absence of rupture. Variables with p < 0.10 were entered into the multivariable logistic regression model with the final model constructed using backward elimination. Adjusted odds ratios (OR) with 95% confidence intervals (CIs) were calculated, and p < 0.05 was considered statistically significant. A subgroup analysis among current and former smokers, including duration and intensity of smoking, and duration since smoking cessation was also performed. Multiple imputation with chained equations was used to account for missing values. Inferential statistics was obtained from 40 imputed datasets. Sensitivity analyses were also performed using subgroups consisting of (1) complete cases only and (2) prospective cases only. All statistical analyses were performed using STATA 14 (STATACorp., College Station, TX).
Standard protocol approvals, registrations, and patient consents.
Institutional review board approval was obtained for this study.
RESULTS
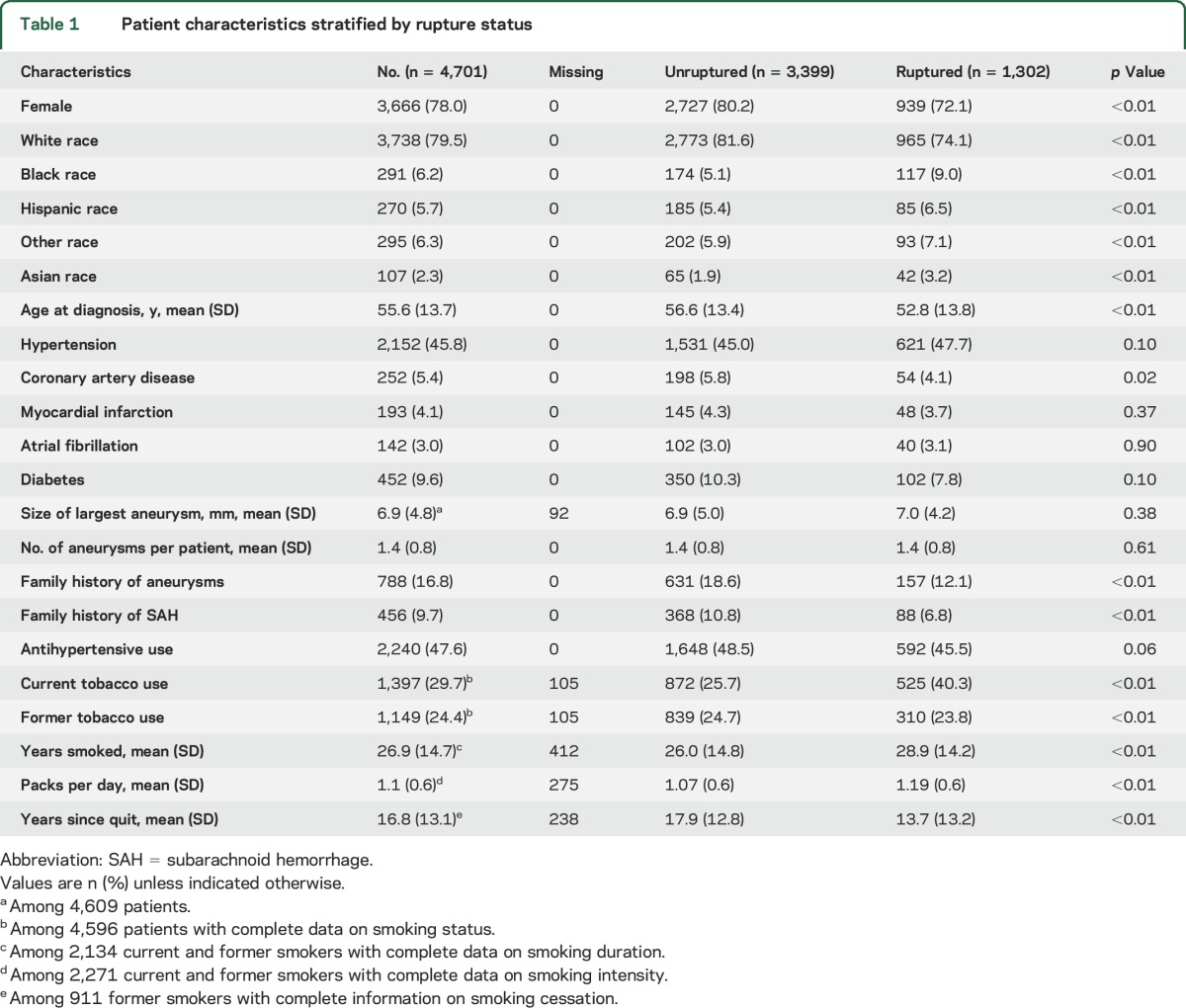
In the 4,701 patients evaluated, there were 6,411 IAs. Of these patients, 1,302 (27.7%) presented with a ruptured aneurysm. Baseline characteristics of patients who presented with unruptured vs ruptured aneurysms are summarized in table 1. In the unruptured group, IAs were identified incidentally in cerebrovascular imaging (CT angiography, magnetic resonance angiography, or cerebral angiogram) during evaluations for headache or dizziness, stroke, neurologic symptoms, trauma, seizures, aneurysm screening due to positive family history, or screening for cancer or other diseases. Patients with ruptured aneurysms were more frequently male, younger, and nonwhite, compared with the unruptured group. In addition, family history of aneurysms or aSAH was significantly associated with unruptured aneurysms.
Table 1.
Patient characteristics stratified by rupture status
The results of the univariable and multivariable analyses in all patients and in a subgroup of former and current smokers are shown in tables 2 and 3, respectively. In the univariable analysis for the entire cohort, current tobacco use (OR 2.29, 95% CI 1.97–2.67) and former tobacco use (OR 1.41, 95% CI 1.19–1.67) compared with never smokers were significantly associated with aneurysm rupture at presentation. In a subgroup analysis of 2,546 former and current smokers, current vs former smoking (OR 1.63, 95% CI 1.38–1.93), years smoked (OR 1.01, 95% CI 1.01–1.02), and packs of cigarettes per day (OR 1.39, 95% CI 1.20–1.60) were significantly associated with aneurysm rupture at presentation. In contrast, a longer period of smoking cessation was associated with a significantly lower risk of aneurysm rupture (OR 0.98, 95% CI 0.97–0.98). Although the size of some coefficients changed minimally, the direction and significance of tobacco-related measures remained the same in the sensitivity analyses using complete cases only and prospective cases only (tables e-1 and e-2 at Neurology.org).
Table 2.
Univariable and multivariable logistic regression for rupture status in current, former, and never smokers (n = 4,701)
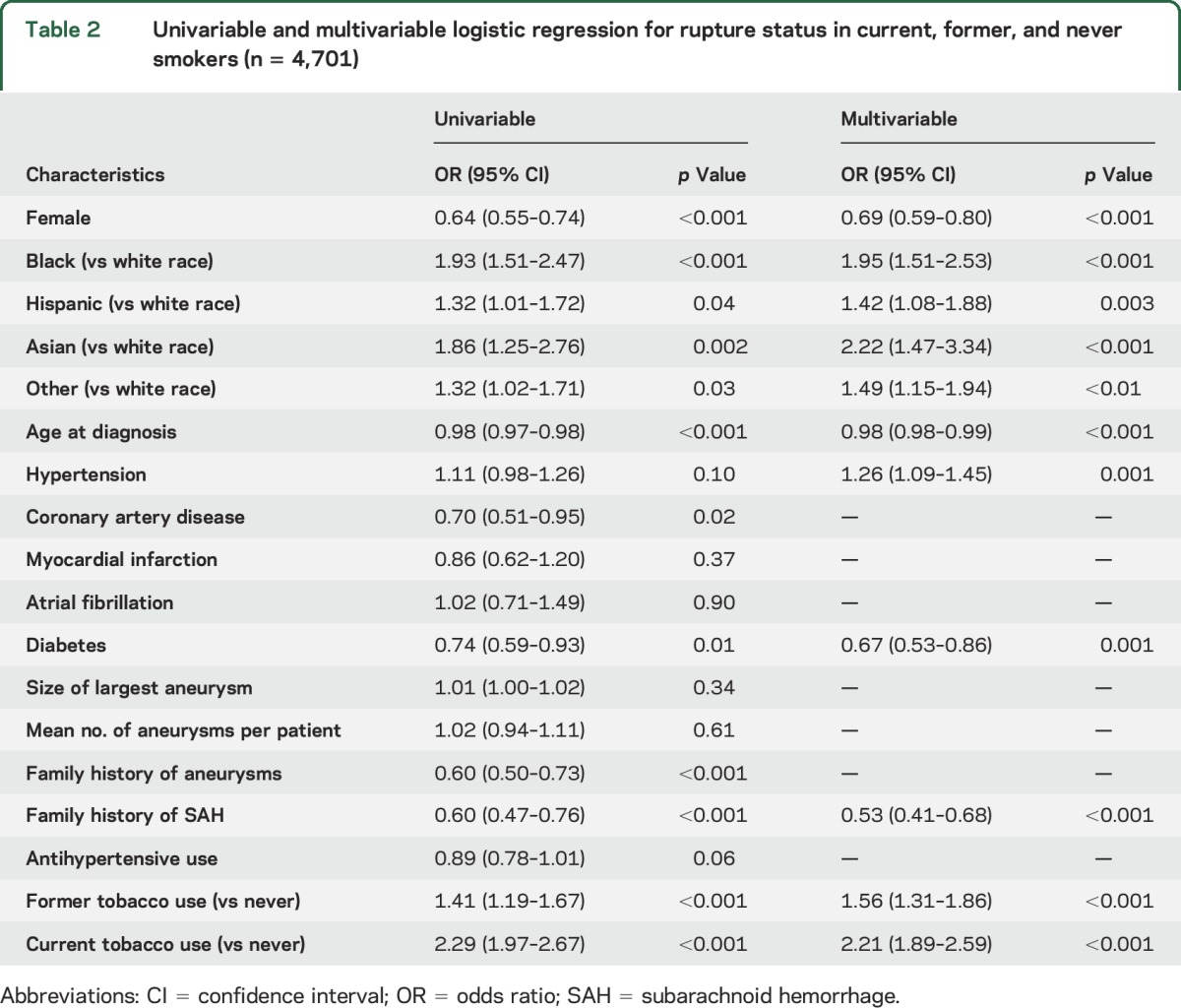
Table 3.
Univariable and multivariable logistic regression for rupture status in former and current smokers (n = 2,546)
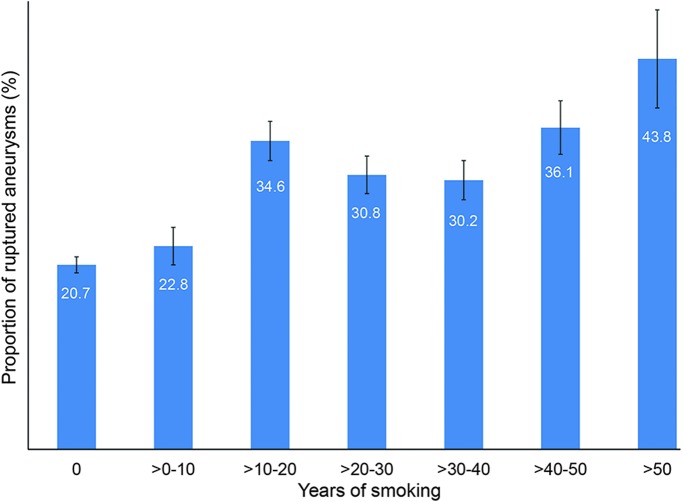
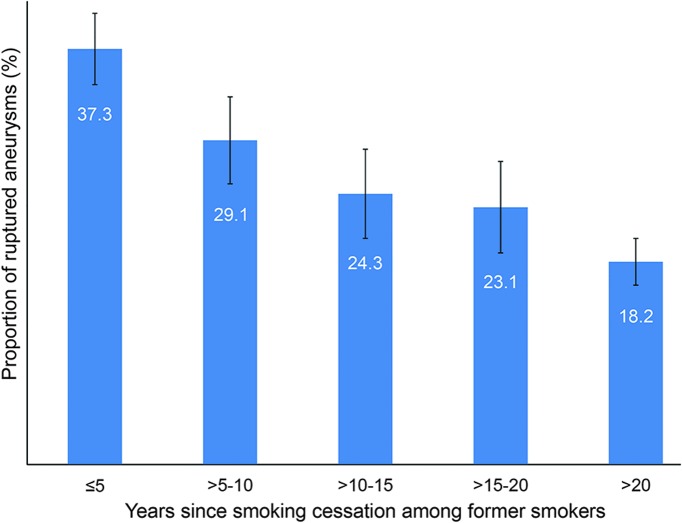
In the multivariable analysis in the full cohort (i.e., current, former, and never smokers), black (OR 1.95, 95% CI 1.51–2.53), Hispanic (OR 1.42, 95% CI 1.08–1.88), Asian (OR 2.22, 95% CI 1.47–3.34), and other races (OR 1.49, 95% CI 1.15–1.94) compared with white race, hypertension (OR 1.26, 95% CI 1.09–1.45), current tobacco use (OR 2.21, 95% CI 1.89–2.59), and former tobacco use (OR 1.56, 95% CI 1.31–1.86) compared with never smokers were associated with increased risk of aneurysm rupture at presentation. Younger age (OR 0.98, 95% CI 0.98–0.99), female sex (OR 0.69, 95% CI 0.59–0.80), diabetes (OR 0.67, 95% CI 0.53–0.86), and family history of subarachnoid hemorrhage (SAH) (OR 0.53, 95% CI 0.41–0.68) were associated with a reduced risk of IA rupture. In a subgroup analysis of 2,546 former and current smokers, years smoked (OR 1.02, 95% CI 1.01–1.03) and packs per day (OR 1.46, 95% CI 1.25–1.70) were significantly associated with aneurysm rupture. Figures 1 and 2 show a dose-dependent increase in the proportion of ruptured aneurysms stratified according to number of packs per day and years smoked, respectively. Figure 3 shows a decrease in the proportion of ruptured aneurysms according to years of cessation among former smokers. However, years since smoking cessation lost significance in the multivariable analysis. The direction and significance of smoking-related coefficients remained the same in the sensitivity analyses using complete cases only (tables e-1 and e-2). Although the amount of missing data on tobacco use (2.5% retrospective vs 0.6% prospective, p < 0.01) and smoking duration (17.3% retrospective vs 11.6% prospective, p < 0.01) were significantly less in the prospective cohort, subgroup analysis in the prospective cohort showed comparable results regarding current vs never smokers (OR 1.87, 95% CI 1.28–2.73), former vs never smokers (OR 1.48, 95% CI 1.02–2.15), years smoked (OR 1.02, 95% CI 1.00–1.04), packs per day (OR 2.07, 95% CI 1.37–3.13), and years since quit (OR 0.99, 95% CI 0.96–1.01) (tables e-1 and e-2).
Figure 1. Proportion of ruptured aneurysms stratified according to number of packs per day smoked.
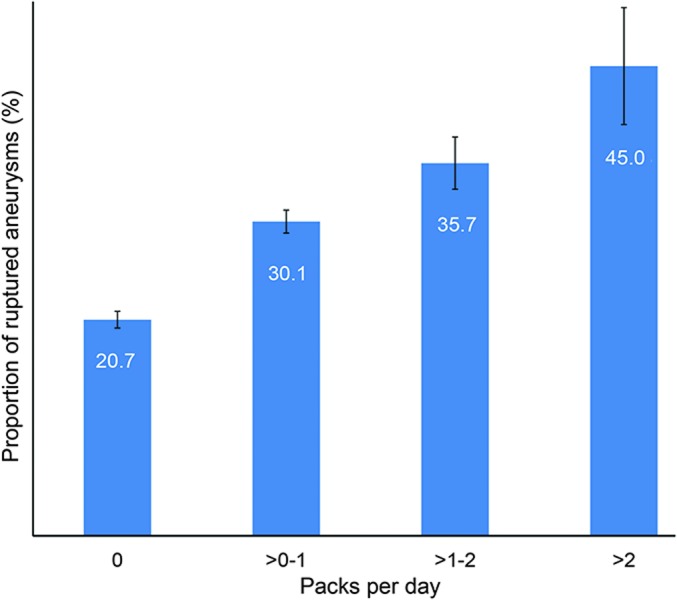
Error bars are standard errors.
Figure 2. Proportion of ruptured aneurysms stratified according to years smoked.
Error bars are standard errors.
Figure 3. Proportion of ruptured aneurysms stratified according to years since tobacco cessation among former smokers.
Error bars are standard errors.
DISCUSSION
Our study, one of the largest studies on the effect of cigarette smoking on IA rupture to date, demonstrates that current and former smoking is significantly associated with the presence of ruptured aneurysms at initial presentation. Current smokers have a 2.5-fold odds of rupture whereas former smokers have an almost 2-fold odds of rupture compared with never smokers. These findings are in line with a prior meta-analysis in which the odds of rupture in former smokers was twice that of never smokers (OR 2.3, 95% CI 2.2–2.4), and the odds for rupture in current smokers was 3 times that of never smokers (OR 3.1, 95% CI 2.7–3.5) in case-control studies.33 However, in contrast to our study, the previous studies that assessed the risk of smoking on IA rupture did not include controls with unruptured IAs, and included healthy controls instead. In addition, the presence of ruptured aneurysms was not confirmed in 12 previous studies, leaving the possibility of misclassification bias due to inclusion of traumatic or other non-aSAH cases.4–7,9,10,12–14,23,26,29 Moreover, the number of included patients with confirmed aneurysm rupture was small in most studies, ranging from 2621 to 42616 patients with aSAH in case-control studies, and from 12211 to 1328 confirmed aSAH cases in longitudinal studies. Furthermore, most studies did not control for antihypertensive medication use, family history of aneurysms and SAH, and aneurysm-related factors such as size and multiplicity. None of the prior studies included duration of smoking in years smoked, and only 2 case-control studies16,28 and 1 longitudinal study4 assessed the cumulative effects of cigarette smoking on aneurysm rupture risk by means of inclusion of pack-years. In a recent Korean multicenter case-control study with 426 nontraumatic SAH cases, the authors demonstrated a dose-dependent increase in risk based on cumulative dose of smoking (e.g., pack-years). However, our study is the first to assess models with smoking intensity and duration as separate variables, instead of combined in pack-years and intensity, in order to evaluate the effects of duration and intensity separately. We showed that a higher dose rate (e.g., number of packs smoked per day) among current and former smokers was significantly associated with an increased risk of IA rupture, whereas the effect of smoking duration had a smaller effect size, but was still significant. Interestingly, although a longer duration since smoking cessation was associated with decreased risk of aneurysm rupture among former smokers in univariable analysis, this association disappeared after adjustment for other confounders in the multivariable analysis. These results suggest that the increased risk of aSAH with smoking persists even after the cessation of cigarette smoking, and that early cessation does not confer a reduced risk of SAH beyond that of reducing the cumulative dose. Although several previous studies demonstrated a significant increased risk of SAH among previous smokers compared to never smokers,8,9,21,22,29 only one of these studies investigated if cessation duration would affect this risk.9 In contrast to our results, one study concluded that the age-adjusted risk ratio of SAH among former smokers returned to the level of never smokers after 5–9 years of cessation. However, that study included only white middle-aged women without confirmation of the presence of ruptured aneurysms, and the number of former smokers with SAH was only 25, limiting its statistical power and validity.9 Five other studies demonstrated that smoking cessation decreases the risk of SAH in a time-dependent manner.4,5,15,16,20 Of the 5 studies, the presence of aSAH was not confirmed in 2 of the studies4,5 and 1 study failed to show increased risk reduction in previous heavy smokers.16 All 5 studies utilized healthy controls with no aneurysms instead of controls with unruptured aneurysms, which likely affected the conclusions drawn.4,5,9,15,16,20
Several theories have been proposed to explain the mechanisms by which cigarette smoking leads to IA formation and rupture.34 Using a 3D model, it was recently demonstrated that smoking is associated with an increase in wall shear stress at the site of aneurysm initiation, probably by increasing blood viscosity and volume, and by inducing cerebral vasoconstriction.34,35 Several authors have attributed the vasoconstrictive effects of nicotine to impaired nitric oxide signaling, an important factor in regulating vascular tone.36–38 In addition, others have recently demonstrated by using an in vitro model using rat cerebral arteries that smoke particles induce upregulation of endothelin type B receptors, which play a critical role in aneurysm pathogenesis through activation of key intracellular inflammatory signal molecules.39 This proinflammatory activation of endothelial cells due to cigarette smoking leads to disruption of their normal function and is thought to result in aneurysm formation and rupture.39 Our observation that the risk of aSAH among former smokers persists after smoking cessation could be explained by these structural and to a certain extent irreversible changes. However, since the risk of aSAH among previous smokers was substantially lower compared to current smokers, and in light of the clear dose–response relation between cigarette smoke and aSAH, our results emphasize the importance of smoking cessation in patients harboring unruptured IAs.
The main strengths of our study are the large sample size, the presence of a large control group with unruptured IAs, and the use of a high-quality homogeneous database. The main limitations of our study include the partially retrospective design, the restriction of our conclusions to the timepoint of initial presentation, and self-reporting of smoking status. However, self-report of tobacco use has previously been demonstrated to be a valid approach to determining tobacco status.40 Nevertheless, in some cases of aSAH, history of cigarette smoking was obtained from relatives of patients in poor clinical condition, which could have led to information bias. Even though this is one of the largest studies on the risk of cigarette smoking on IA rupture to date, causality between cigarette smoking and IA rupture will need to be established in future mechanistic studies.
We found that current smoking status, smoking duration, and smoking intensity are the most important risk factors for IA rupture at presentation. In addition, we demonstrated a lower but significantly increased risk of aneurysm rupture among former smokers compared to never smokers, and that early cessation does not confer a reduced risk beyond that of reducing the cumulative dose, suggesting that there are irreversible effects due to cigarette smoking.
Supplementary Material
GLOSSARY
- aSAH
aneurysmal subarachnoid hemorrhage
- BWH
Brigham and Women's Hospital
- CI
confidence interval
- IA
intracranial aneurysm
- ICD-9
International Classification of Diseases–9
- MGH
Massachusetts General Hospital
- NLP
natural language processing
- OR
odds ratio
- RPDR
Partners Healthcare Research Patients Data Registry
- SAH
subarachnoid hemorrhage
Footnotes
Supplemental data at Neurology.org
Editorial, page 1320
AUTHOR CONTRIBUTIONS
Anil Can: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content. Victor M. Castro: analysis and interpretation of data, critical revision of manuscript for intellectual content. Yildirim H. Ozdemir: acquisition of data, critical revision of manuscript for intellectual content. Sarajune Dagen: acquisition of data, critical revision of manuscript for intellectual content. Sheng Yu: analysis and interpretation of data, critical revision of manuscript for intellectual content. Dmitriy Dligach: analysis and interpretation of data, critical revision of manuscript for intellectual content. Sean Finan: analysis and interpretation of data, critical revision of manuscript for intellectual content. Vivian Gainer: analysis and interpretation of data, critical revision of manuscript for intellectual content. Nancy A. Shadick: analysis and interpretation of data, critical revision of manuscript for intellectual content. Shawn Murphy: analysis and interpretation of data, critical revision of manuscript for intellectual content. Tianxi Cai: analysis and interpretation of data, critical revision of manuscript for intellectual content. Guergana Savova: analysis and interpretation of data, critical revision of manuscript for intellectual content. Ruben Dammers: analysis and interpretation of data, critical revision of manuscript for intellectual content. Scott T. Weiss: analysis and interpretation of data, critical revision of manuscript for intellectual content. Rose Du: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content.
STUDY FUNDING
This study was supported by Partners Personalized Medicine.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997;28:660–664. [DOI] [PubMed] [Google Scholar]
- 2.Woo D, Khoury J, Haverbusch MM, et al. Smoking and family history and risk of aneurysmal subarachnoid hemorrhage. Neurology 2009;72:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability and risk factors for aneurysm rupture. Neurosurg Focus 2000;8:Preview 1. [PubMed] [Google Scholar]
- 4.Lindbohm JV, Kaprio J, Jousilahti P, Salomaa V, Korja M. Sex, smoking, and risk for subarachnoid hemorrhage. Stroke 2016;47:1975–1981. [DOI] [PubMed] [Google Scholar]
- 5.Korja M, Silventoinen K, Laatikainen T, et al. Risk factors and their combined effects on the incidence rate of subarachnoid hemorrhage: a population-based cohort study. PLoS One 2013;8:e73760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suh I, Jee SH, Kim HC, Nam CM, Kim IS, Appel LJ. Low serum cholesterol and haemorrhagic stroke in men: Korea Medical Insurance Corporation Study. Lancet 2001;357:922–925. [DOI] [PubMed] [Google Scholar]
- 7.Kurth T, Kase CS, Berger K, Schaeffner ES, Buring JE, Gaziano JM. Smoking and the risk of hemorrhagic stroke in men. Stroke 2003;34:1151–1155. [DOI] [PubMed] [Google Scholar]
- 8.Sandvei MS, Romundstad PR, Muller TB, Vatten L, Vik A. Risk factors for aneurysmal subarachnoid hemorrhage in a prospective population study: the HUNT study in Norway. Stroke 2009;40:1958–1962. [DOI] [PubMed] [Google Scholar]
- 9.Kawachi I, Colditz GA, Stampfer MJ, et al. Smoking cessation and decreased risk of stroke in women. JAMA 1993;269:232–236. [PubMed] [Google Scholar]
- 10.Knekt P, Reunanen A, Aho K, et al. Risk factors for subarachnoid hemorrhage in a longitudinal population study. J Clin Epidemiol 1991;44:933–939. [DOI] [PubMed] [Google Scholar]
- 11.Lindekleiv H, Sandvei MS, Romundstad PR, et al. Joint effect of modifiable risk factors on the risk of aneurysmal subarachnoid hemorrhage: a cohort study. Stroke 2012;43:1885–1889. [DOI] [PubMed] [Google Scholar]
- 12.Klatsky AL, Armstrong MA, Friedman GD, Sidney S. Alcohol drinking and risk of hemorrhagic stroke. Neuroepidemiology 2002;21:115–122. [DOI] [PubMed] [Google Scholar]
- 13.Yamada S, Koizumi A, Iso H, et al. Risk factors for fatal subarachnoid hemorrhage: the Japan Collaborative Cohort Study. Stroke 2003;34:2781–2787. [DOI] [PubMed] [Google Scholar]
- 14.Feigin V, Parag V, Lawes CM, et al. Smoking and elevated blood pressure are the most important risk factors for subarachnoid hemorrhage in the Asia-Pacific region: an overview of 26 cohorts involving 306,620 participants. Stroke 2005;36:1360–1365. [DOI] [PubMed] [Google Scholar]
- 15.Anderson CS, Feigin V, Bennett D, et al. Active and passive smoking and the risk of subarachnoid hemorrhage: an international population-based case-control study. Stroke 2004;35:633–637. [DOI] [PubMed] [Google Scholar]
- 16.Kim CK, Kim BJ, Ryu WS, Lee SH, Yoon BW. Impact of smoking cessation on the risk of subarachnoid haemorrhage: a nationwide multicentre case control study. J Neurol Neurosurg Psychiatry 2012;83:1100–1103. [DOI] [PubMed] [Google Scholar]
- 17.Bonita R. Cigarette smoking, hypertension and the risk of subarachnoid hemorrhage: a population-based case-control study. Stroke 1986;17:831–835. [DOI] [PubMed] [Google Scholar]
- 18.Kissela BM, Sauerbeck L, Woo D, et al. Subarachnoid hemorrhage: a preventable disease with a heritable component. Stroke 2002;33:1321–1326. [DOI] [PubMed] [Google Scholar]
- 19.Juvela S, Hillbom M, Numminen H, Koskinen P. Cigarette smoking and alcohol consumption as risk factors for aneurysmal subarachnoid hemorrhage. Stroke 1993;24:639–646. [DOI] [PubMed] [Google Scholar]
- 20.Longstreth WT Jr, Nelson LM, Koepsell TD, van Belle G. Cigarette smoking, alcohol use, and subarachnoid hemorrhage. Stroke 1992;23:1242–1249. [DOI] [PubMed] [Google Scholar]
- 21.Isaksen J, Egge A, Waterloo K, Romner B, Ingebrigtsen T. Risk factors for aneurysmal subarachnoid haemorrhage: the Tromsø study. J Neurol Neurosurg Psychiatry 2002;73:185–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qureshi AI, Suri MF, Yahia AM, et al. Risk factors for subarachnoid hemorrhage. Neurosurgery 2001;49:607–612. [DOI] [PubMed] [Google Scholar]
- 23.Fogelholm R, Murros K. Cigarette smoking and subarachnoid haemorrhage: a population-based case-control study. J Neurol Neurosurg Psychiatry 1987;50:78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okamoto K, Horisawa R, Kawamura T, et al. Family history and risk of subarachnoid hemorrhage: a case-control study in Nagoya, Japan. Stroke 2003;34:422–426. [DOI] [PubMed] [Google Scholar]
- 25.Kubota M, Yamaura A, Ono J. Prevalence of risk factors for aneurysmal subarachnoid haemorrhage: results of a Japanese multicentre case control study for stroke. Br J Neurosurg 2001;15:474–478. [DOI] [PubMed] [Google Scholar]
- 26.Kunze AK, Annecke A, Wigger F, et al. Recent infection as a risk factor for intracerebral and subarachnoid hemorrhages. Cerebrovasc Dis 2000;10:352–358. [DOI] [PubMed] [Google Scholar]
- 27.Ohkuma H, Tabata H, Suzuki S, Islam MS. Risk factors for aneurysmal subarachnoid hemorrhage in Aomori, Japan. Stroke 2003;34:96–100. [DOI] [PubMed] [Google Scholar]
- 28.Adamson J, Humphries SE, Ostergaard JR, Voldby B, Richards P, Powell JT. Are cerebral aneurysms atherosclerotic? Stroke 1994;25:963–966. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Rodriguez LA, Gaist D, Morton J, Cookson C, Gonzalez-Perez A. Antithrombotic drugs and risk of hemorrhagic stroke in the general population. Neurology 2013;81:566–574. [DOI] [PubMed] [Google Scholar]
- 30.Broderick JP, Viscoli CM, Brott T, et al. Major risk factors for aneurysmal subarachnoid hemorrhage in the young are modifiable. Stroke 2003;34:1375–1381. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Sandoval JL, Cantu C, Chiquete E, et al. Aneurysmal subarachnoid hemorrhage in a Mexican multicenter registry of cerebrovascular disease: the RENAMEVASC study. J Stroke Cerebrovasc Dis 2009;18:48–55. [DOI] [PubMed] [Google Scholar]
- 32.Castro VM, Dligach D, Finan S, et al. Large-scale identification of patients with cerebral aneurysms using natural language processing. Neurology 2017;88:164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feigin VL, Rinkel GJ, Lawes CM, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 2005;36:2773–2780. [DOI] [PubMed] [Google Scholar]
- 34.Chalouhi N, Ali MS, Starke RM, et al. Cigarette smoke and inflammation: role in cerebral aneurysm formation and rupture. Mediators Inflamm 2012;2012:271582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh PK, Marzo A, Howard B, et al. Effects of smoking and hypertension on wall shear stress and oscillatory shear index at the site of intracranial aneurysm formation. Clin Neurol Neurosurg 2010;112:306–313. [DOI] [PubMed] [Google Scholar]
- 36.Mayhan WG, Patel KP. Effect of nicotine on endothelium-dependent arteriolar dilatation in vivo. Am J Physiol 1997;272:H2337–H2342. [DOI] [PubMed] [Google Scholar]
- 37.Koide M, Nishizawa S, Yamamoto S, Yamaguchi M, Namba H, Terakawa S. Nicotine exposure, mimicked smoking, directly and indirectly enhanced protein kinase C activity in isolated canine basilar artery, resulting in enhancement of arterial contraction. J Cereb Blood Flow Metab 2005;25:292–301. [DOI] [PubMed] [Google Scholar]
- 38.Gerzanich V, Zhang F, West GA, Simard JM. Chronic nicotine alters NO signaling of Ca(2+) channels in cerebral arterioles. Circ Res 2001;88:359–365. [DOI] [PubMed] [Google Scholar]
- 39.Sandhu H, Xu CB, Edvinsson L. Upregulation of contractile endothelin type B receptors by lipid-soluble cigarette smoking particles in rat cerebral arteries via activation of MAPK. Toxicol Appl Pharmacol 2010;249:25–32. [DOI] [PubMed] [Google Scholar]
- 40.Yeager DS, Krosnick JA. The validity of self-reported nicotine product use in the 2001–2008 National Health and Nutrition Examination Survey. Med Care 2010;48:1128–1132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.