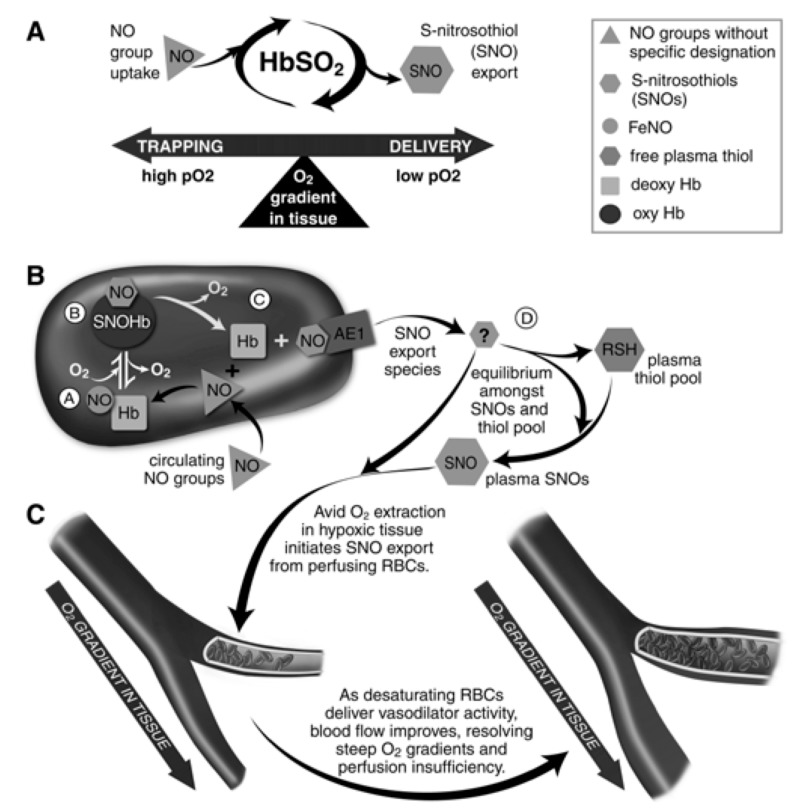
Figure 6.
Red blood cells (RBC) transduce regional O2 gradients in tissue to control nitric oxide (NO) bioactivity in plasma by trapping or delivering NO groups as a function of haemoglobin (Hb) O2 saturation (Hb SO2).
(A) Circulating NO groups are processed by Hb into the highly vasoactive (thiol-based) NO congener, S-nitrosothiol (SNO). By exporting SNOs as a function of Hb deoxygenation, RBCs precisely dispense vasodilator bioactivity in direct proportion to the lack of regional blood flow. (B) O2 delivery homeostasis requires biochemical coupling of vessel tone to environmental cues that match perfusion sufficiency to metabolic demand. Because oxygenated Hb (oxy Hb) and deoxygenated Hb (deoxy Hb) process NO differently, allosteric transitions in Hb conformation afford context-responsive (O2-coupled) control of NO bioavailability, thereby linking the sensor and effector arms of this system. Specifically, Hb conformation governs the equilibria among (A) deoxygenated Hb FeNO (NO sink), (B) oxygenated SNO-Hb (NO store), and (C) acceptor thiols including the membrane protein SNO-AE-1 (bioactive NO source). Direct SNO export from RBCs or S-transnitrosylation from RBCs to plasma thiols (D) yields vasoactive SNOs, which influence resistance vessel caliber and close this signalling loop. Thus, RBCs either trap (A) or export (D) NO groups to optimise blood flow. (C) NO processing in RBCs (A and B) couples vessel tone to tissue PO2; this system subserves hypoxic vasodilation in the arterial periphery and thereby calibrates blood flow to regional tissue hypoxia. (Adapted with permission from Doctor and Stamler, 201181. ©American Physiological Society). RSH: peptide or protein containing a thiol group.