Figure 1. M3R activation stimulates p38 MAPK and ERK1/2 phosphorylation.
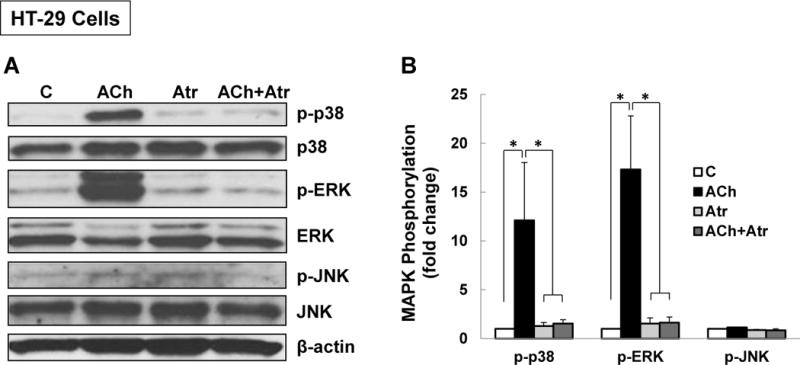
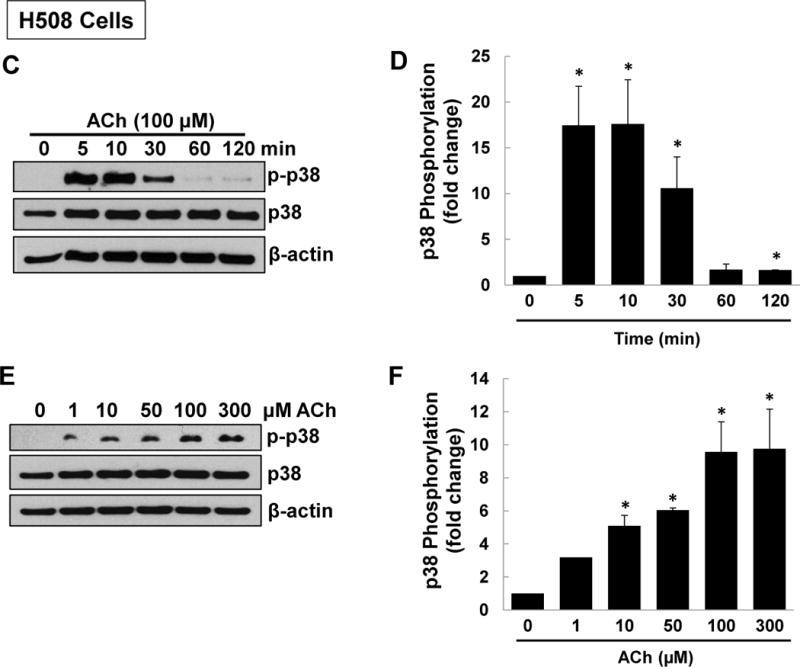
(A) Acetylcholine (ACh) stimulates p38 and ERK1/2 phosphorylation. HT-29 colon cancer cells were incubated for 5 min with 200 μM ACh and cell extracts were immunoblotted with antibodies against phosphorylated and total p38, ERK1/2, and JNK. (B) Densitometry of immunoblots from four separate experiments show that ACh stimulated robust phosphorylation of p38 and ERK1/2, but did not alter levels of total or phosphorylated JNK, total ERK1/2 or total p38. The actions of ACh were blocked by pre-incubating cells with 5 μM atropine (Atr) for 45 min. C, untreated control; *P < 0.05. (C) ACh stimulates time-dependent p38 phosphorylation. H508 colon cancer cells were incubated with 100 μM ACh for the indicated times. Cell extracts were immunoblotted with antibodies against phosphorylated and total p38. (D) Densitometry of immunoblots from three separate experiments show that ACh stimulated maximal p38 phosphorylation within 5 to 10 min which was nearly back to basal levels within 1 h. *P < 0.05 compared to Time 0. (E) ACh stimulates dose-dependent p38 phosphorylation. H508 colon cancer cells were incubated with the indicated concentrations of ACh for 10 min. Cell extracts were immunoblotted with antibodies against phosphorylated and total p38. (F) Densitometry of immunoblots from three separate experiments show ACh-induced p38 phosphorylation was detectable with 1 μM ACh and maximal with 100 to 300 μM ACh. *P < 0.05 compared to vehicle alone (no ACh). In all immunoblots, β-actin was used as a loading control.
