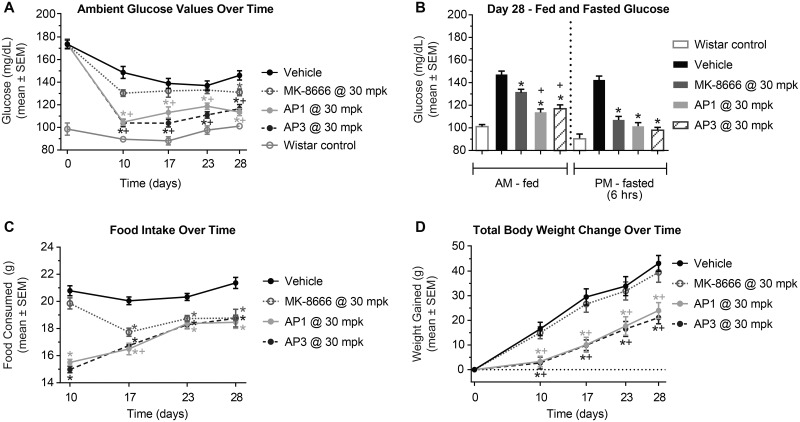
Fig 3. GK rat glucose, body weight, and food intake are affected by 28 days treatment with AgoPAMs or partial agonist.
All compounds were dosed at maximal efficacious doses. Fed blood glucose levels were significantly reduced by GPR40 AgoPAMs compared to vehicle controls on days 10 through 28 of treatment, but only on day 28 by the partial agonist (P < 0.05, A). On day 28 of study, fed and fasted blood glucose was significantly reduced with GPR40 partial and AgoPAM treatments compared to vehicle controls (P < 0.05, B). Additionally, AP1 significantly reduced fed blood glucose compared to the partial agonist (P < 0.05, B). Decreased levels of food intake were observed with AgoPAM treatment days 10 through 28 (P < 0.05), while the partial agonist did not reduce food intake until day 17 (P < 0.05, C). The effects of AgoPAMs on food intake were associated with decreases in body weight (P < 0.05, D) whereas MK-8666 had no effect on body weight. Data are mean ± SEM with analysis via ANOVA followed by Tukey’s posttest. * P < 0.05 vs. Vehicle, + P < 0.05 vs. MK-8666.