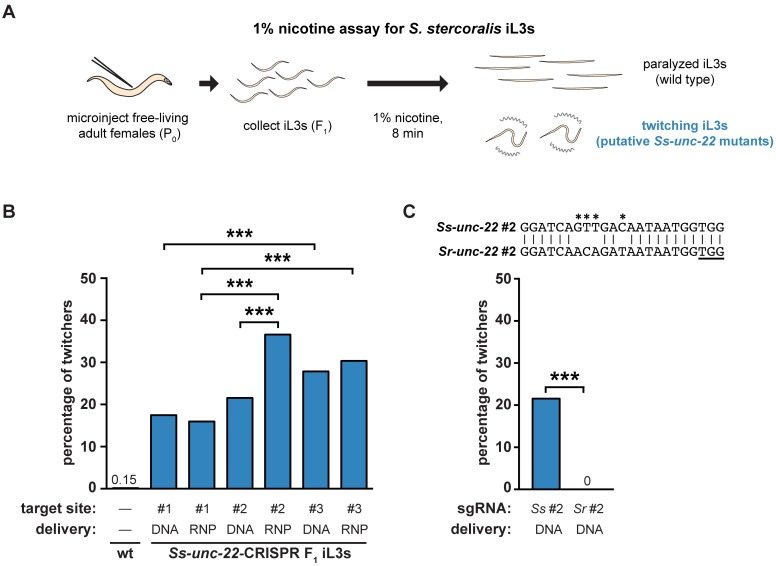
Fig 3. Nicotine induces twitching in unc F1 iL3s.
(A) A nicotine assay for S. stercoralis iL3s. Free-living adult females were injected with CRISPR constructs targeting Ss-unc-22. F1 iL3s were collected and exposed to 1% nicotine. Wild-type iL3s gradually paralyzed over the course of 8 min, whereas unc F1 iL3s twitched continuously. Some, but not all, of the F1 iL3s contained putative Ss-unc-22 mutations and twitched in nicotine. (B) Twitching frequency of S. stercoralis wild-type iL3s and the F1 iL3s from microinjected females following nicotine exposure. For each condition, the Ss-unc-22 target site and delivery method of the CRISPR-Cas9 constructs are indicated. DNA = plasmid vector delivery; RNP = ribonucleoprotein complex delivery. The twitching frequency of F1 iL3s for all Ss-unc-22 target sites and delivery methods tested differed from that of wild-type iL3s (P<0.001, chi-square test with Bonferroni correction). Instances where twitching frequency differed between target sites or delivery methods are indicated. ***P<0.001, chi-square test with Bonferroni correction. n = 446–1,314 iL3s per condition. (C) CRISPR-Cas9-mediated mutagenesis of Ss-unc-22 requires a highly specific sgRNA. Plasmid vectors for the expression of Cas9 and a sgRNA targeting S. ratti site #2 were injected into S. stercoralis. The twitching phenotype in S. stercoralis F1 iL3s was not observed when the S. ratti version of site #2 was used. ***P<0.001, Fisher’s exact test. n = 484-677 iL3s for each condition. The alignment of S. stercoralis and S. ratti site #2 is shown with the PAM underlined. Asterisks indicate nucleotide differences between the S. stercoralis and S. ratti targets.