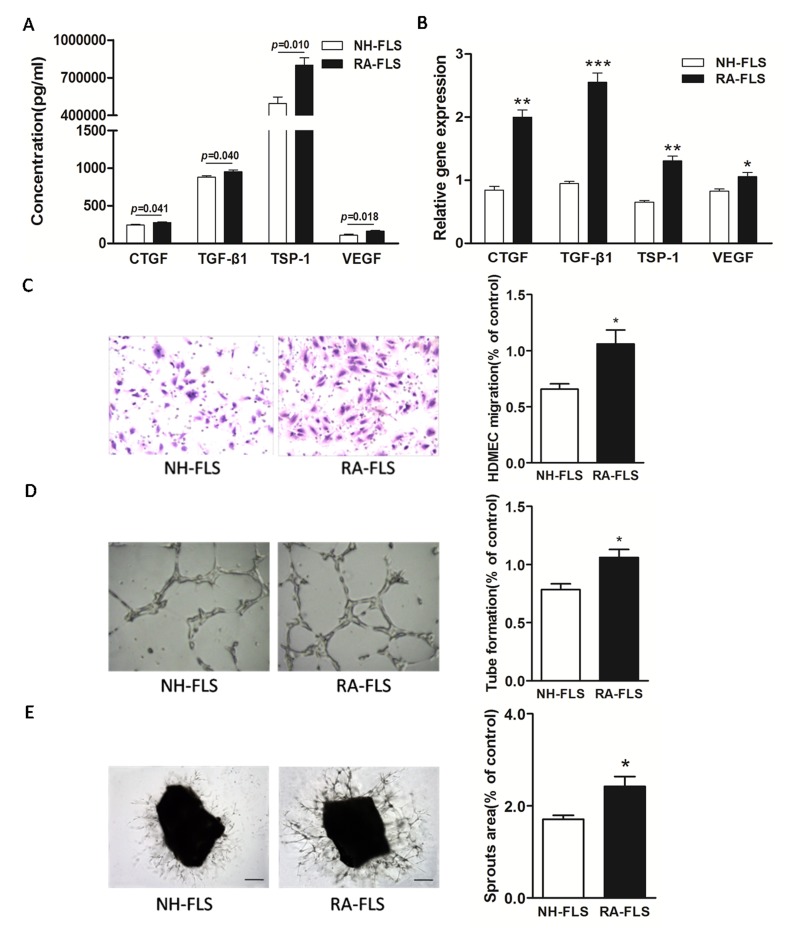
Figure 1. Increased expression of TSP-1, TGF-β1, CTGF and VEGF in supernatants of RA-FLS and HDMECs co-culture compared to NH-FLS and HDMECs co-culture.
Normal human (NH) FLS and rheumatoid arthritis (RA) FLS were co-cultured with HDMECs for 48 h, respectively. A. ELISA analysis demonstrated significant increase in the concentrations of TSP-1, TGF-β1, CTGF and VEGF in supernatants of RA-FLS and HDMECs co-culture (n = 3) compared with those from NH-FLS and HDMECs co-culture (n = 3; p < 0.05). B. Real-time PCR analysis showed increased mRNA expression of TSP-1, TGF-β1, CTGF and VEGF in RA-FLS co-cultured (n = 3) compared to NH-FLS co-cultured (n = 3; *p < 0.05, **p < 0.01, ***p < 0.001). C. and D. Transwell assay (C; n = 3) and tube formation test (D; n = 3) for 6 h demonstrated significant up-regulation in migration and capillary-like structure formation of HDMECs respectively under treatment of supernatants from RA-FLS and HDMECs co-culture (n = 3) compared to those from NH-FLS and HDMECs co-culture (n = 3; *p < 0.05). E. Mouse aortic rings were placed on GFR-Matrigel-coated plates and incubated in 1% FBS EGM-2. On 3rd day, the EGM-2 were exchanged with supernatants from FLS and HDMECs co-cuture and further incubated for 3 days. Ex vivo aortic ring angiogenesis assay showed significant up-regulation in microvessel sprouting under treatment of supernatants from RA-FLS and HDMECs co-culture (n = 3) compared to those from NH-FLS and HDMECs co-culture (n = 3; *p < 0.05). Bars = 300 μm. Original magnification = ×5. Results are expressed as the mean ± S.E.M.