Figure 2. BPIS prevented nuclear translocation of NF-κB in LPS treated HT-29 cells.
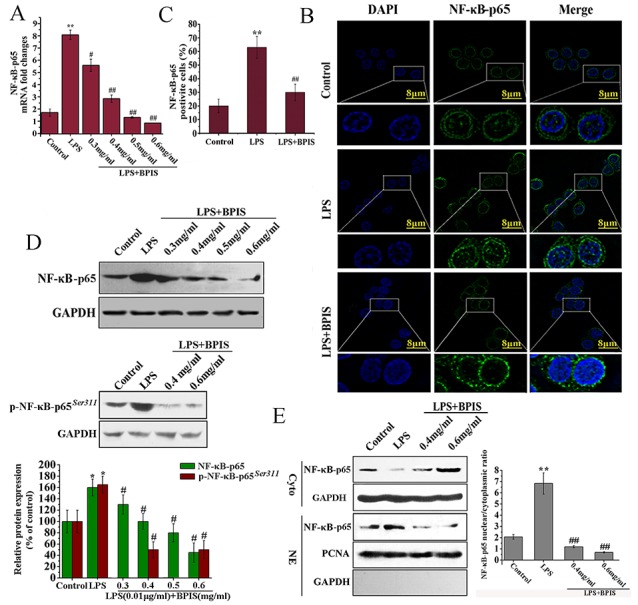
(A) Cells were stimulated with LPS (0.01 μg/ml) for 24 h, and then treated with or without various concentrations of BPIS (0.3 mg/ml, 0.4 mg/ml, 0.5 mg/ml and 0.6 mg/ml) for 24 h. MRNA levels of NF-κB-p65 were determined by RT–PCR using internal control (GAPDH). Data were presented as mean ± SEM (n=3, **p<0.01 vs. control, # p<0.05 vs. LPS, ## p<0.01 vs. LPS) (B) Cells were incubated with or without BPIS (0.6 mg/ml) in the presence of LPS (0.01 μg/ml) for 24 h. Green staining of NF-κB-p65 was present in the nucleus but absent in the cytoplasm of HT-29 cells treated for 24 h with 0.01 μg/ml LPS alone. Dominant green cytoplasmic localization of NF-κB-p65 was observable in cells treated by adding LPS with 0.6 mg/ml BPIS. (C) The percentage of NF-κB-p65 positive cells in ten random fields of the same size. Data were presented as mean ± SEM (n=5, **p<0.01 vs. control, ## p<0.01 vs. LPS). (D) Western blot to evaluate the expression level of NF-κB-p65 and p-NF-κB-p65(Ser311) in cell lysates using relevant antibodies. GAPDH was used as a standard control. Data were presented as mean ± SEM (n=3, *p<0.05 vs. control, # p<0.05 vs. LPS). (E) The cells untreated and treated LPS alone or treated by adding LPS with 0.6 mg/ml BPIS. Western blot assays of NF-κB-p65 expression in nuclear and cytosolic. PCNA and GAPDH were the loading controls, and the presence of GAPDH in nuclear extracts illustrated whether cytoplasmic protein was contaminated. Data were presented as mean ± SEM (n=3, **p<0.01 vs. control, ## p<0.01 vs. LPS).
