Fig. 3. Galvanostatic cycling of symmetric cells using 3D Li-rGO with flowable interphase and planar Li foil electrodes at 60°C.
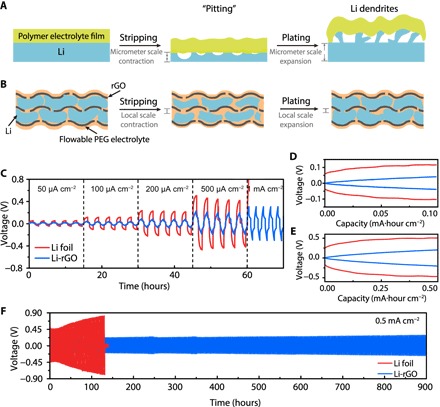
(A) Schematic illustrating the micrometer-scale volume change and uneven Li stripping/plating of Li foil anode, which make it challenging for the solid electrolyte to maintain a continuous contact during cycling. As a “hostless” electrode, the volume of the electrode contracts and expands during Li stripping and plating, respectively. For a practical battery, the areal capacity of a single-sided electrode is ~3 mA·hour cm−2, equivalent to a relative change in thickness of ~15 μm for Li. Moreover, Li tends to be cycled in a nonuniform fashion as localized stripping (pitting) and dendritic plating are often observed. The nonuniform, micrometer-scale electrode-electrolyte interphase movement prevents the formation of a good contact. (B) Schematic illustrating the advantages of the 3D Li-rGO anode for solid Li batteries. The significantly reduced interfacial fluctuation due to increased Li surface area and the flowable nature of the interphase polymer electrolyte are beneficial in maintaining an intimate electrode-electrolyte contact during cycling. (C) Voltage profiles at different current densities. The charging/discharging time was fixed at 1 hour for all the current densities except 1 mA cm−2 (30-min charging/discharging) and with 30-min rest in between. (D and E) Detailed voltage profiles at a current density of 0.1 and 0.5 mA cm−2, respectively. (F) Comparison of the long-term cycling stability of the symmetric cells with Li-rGO electrodes and Li foil electrodes at a current density of 0.5 mA cm−2.
