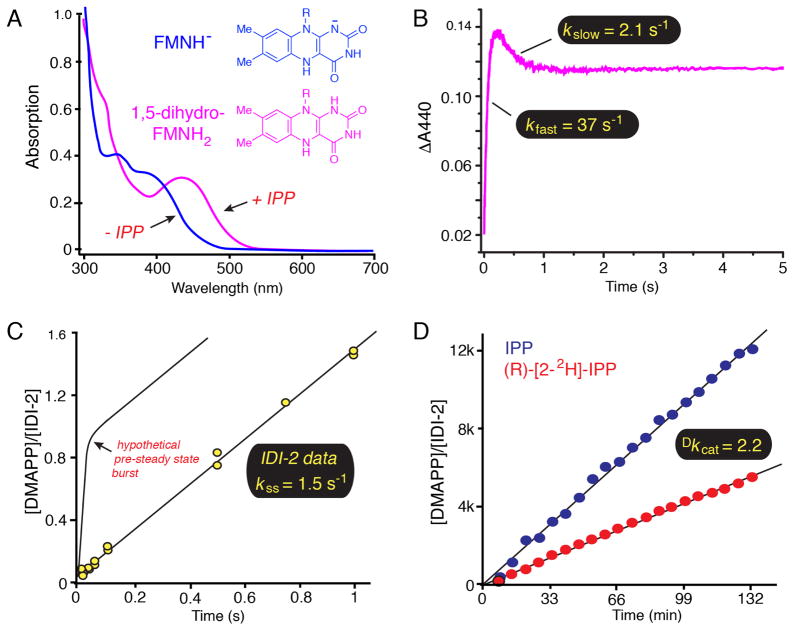
Figure 1.
Biochemical studies of the Staphylococcus aureus IDI-2 enzyme. A) UV-visible absorption spectra of reduced IDI-2:FMN in the absence (blue) and presence (magenta) of IPP. The λmax value of ~ 425 nm in the IDI-2:FMN:IPP complex is consistent with the neutral 1,5-dihydro-FMNH2 tautomeric state of the reduced flavin. B) Pre-steady state accumulation and decay of the FMNH2 intermediate under single turnover conditions. After the rapid accumulation phase (kfast = 37 s−1), the intermediate decays to an equilibrium level at a kinetically competent rate (kslow = 2.1 s−1 > kcat = 1.3 s−1), suggesting that the FMNH2 species could be catalytically relevant. C) Pre-steady state burst experiment. The lack of a detectable pre-steady state burst in DMAPP formation suggests that the rate-determining step in the kinetic mechanism occurs prior to or concomitant with DMAPP formation, yielding a velocity (kss = 1.5 s−1) that is very similar to kcat (1.3 s−1). This observation is consistent with rate-limiting isomerization chemistry step(s). Please note that the theoretical maximum burst amplitude of 1.0 would not be expected for the isomerization reaction catalyzed by IDI-2 where IPP and DMAPP would be in equilibrium at the active site. The depicted curve is only meant to illustrate the diagnostic utility of a pre-steady state burst experiment. D) An 1H-NMR assay was used to measure a primary substrate deuterium kinetic isotope effect on the reaction velocity at saturating IPP concentrations as an approximation of Dkcat. The observed isotope effect (Dkcat = 2.2) is consistent with cleavage of the IPP pro-R C2-H/D bond in a partially rate-determining step.