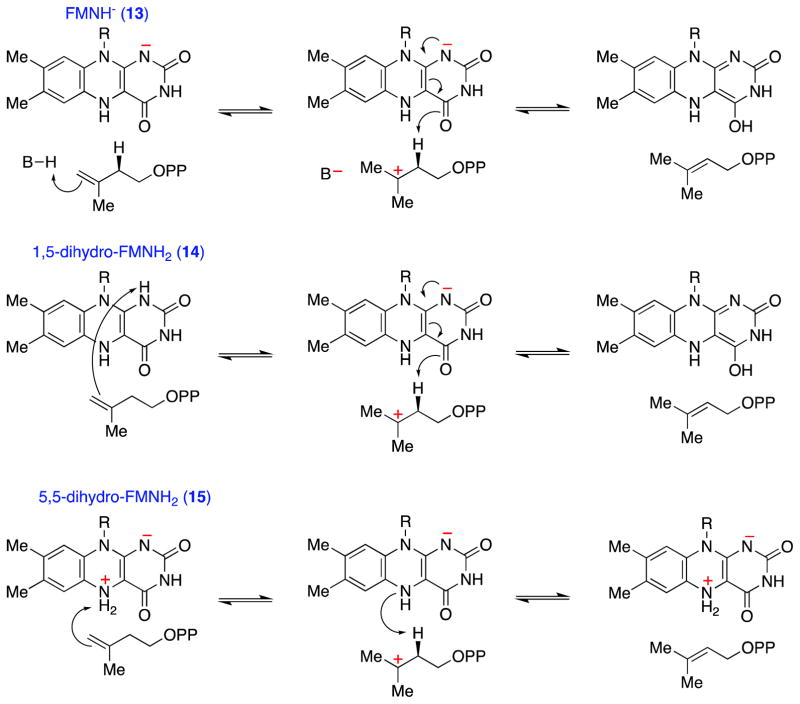
Scheme 7.
General considerations for IDI-2 catalyzed acid/base chemical mechanisms. Various roles for the reduced flavin in acid/base catalysis can be envisioned. The anionic flavin (13) could work in concert with an IDI-2 derived amino acid side chain (top path), or FMNH2 could catalyze both proton transfers via the neutral 1,5-dihydro tautomer (14, middle path) or the zwitterionic 5,5-dihydro tautomer (15, bottom path). The reactions are drawn as occurring by stepwise mechanisms, but the paths involving FMNH− (top) and 1,5-dihydro-FMNH2 (middle) could also occur by concerted proton addition/elimination mechanisms.