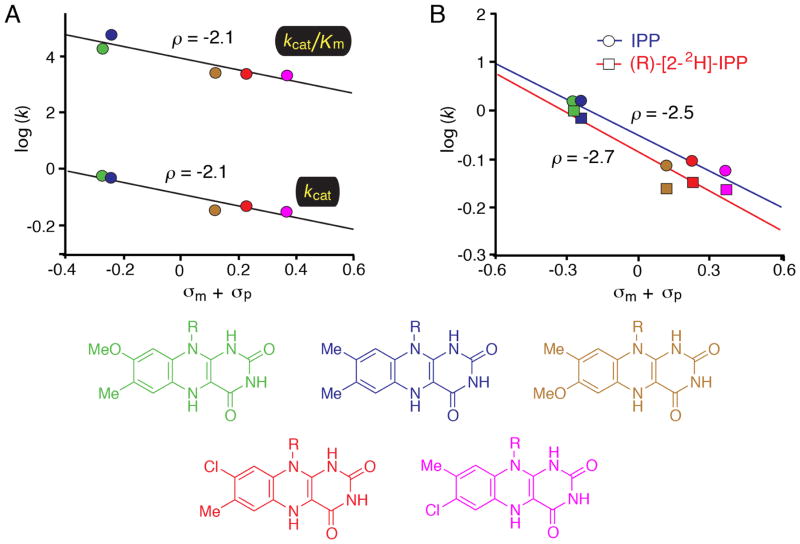
Figure 2.
Linear free energy relationship (LFER) studies of IDI-2. A) Hammett plots correlating the steady state kinetic parameters of IDI-2 enzymes reconstituted with the flavins shown at the bottom of the figure. LFERs were observed when the rate constants were plotted vs. either the sum of the Hammett σm and σp substituent constants or the estimated pKa of the N5 position of the flavin (data not shown). The negative slopes in the Hammett plot (ρ = −2.1) are consistent with the accumulation of positive charge on the flavin N5 atom in the transition state(s) that limit steady state turnover. B) Combined KIE/LFER study. The steady state reaction velocities for IDI-2 reconstituted with each flavin analogue were measured at saturating concentrations of either IPP or (R)-[2-2H]-IPP. The KIE was found to be approximately constant across the series, suggesting that the flavin analogues are neither perturbing the transition state structure nor altering the expression of the KIE by exerting effects on the energetic barriers of other steps in the mechanism.