Summary
Group B Streptococcus (GBS) or Streptococcus agalactiae are β-hemolytic, Gram-positive bacteria that are a leading cause of neonatal infections. GBS commonly colonize the lower gastrointestinal and genital tracts and during pregnancy, neonates are at risk for infection. Although intrapartum antibiotic prophylaxis during labor and delivery has decreased the incidence of early onset neonatal infection, these measures do not prevent ascending infection that can occur earlier in pregnancy leading to preterm births, stillbirths, or late onset neonatal infections. Prevention of GBS infection in pregnancy is complex and likely influenced by multiple factors including pathogenicity, host factors, vaginal microbiome, false negative screening, and/or changes in antibiotic resistance. A deeper understanding of mechanisms of GBS infections during pregnancy will facilitate the development of novel therapeutics and vaccines. Here, we summarize and discuss important advancements in our understanding of GBS vaginal colonization, ascending infection and preterm birth.
Keywords: Group B Streptococcus, Vaginal Colonization, Ascending Infection, Preterm Birth, Perinatal Infection
Infections by Group B Streptococcus During Pregnancy
Group B Streptococcus (GBS) or Streptococcus agalactiae, is a leading cause of infection during pregnancy, preterm birth and neonatal infection [1–3]. GBS was first identified in 1887 as a cause of bovine mastitis [4], and later was isolated from the human vagina [5] and associated with cases of human disease [6]. Subsequently, GBS vaginal colonization was identified as a risk factor for the development of neonatal GBS disease [7, 8] and preterm birth [2, 3]. Women who are vaginally colonized during pregnancy are at risk for ascending infection or transmission of GBS to the newborn during delivery. Ascending infection is a widely-accepted route by which vaginal bacteria move from the vagina, through the cervix and into the uterus and penetrate gestational tissues (Figure 1). Once GBS has invaded the amniotic cavity or come into contact with the placenta, there is the potential for chorioamnionitis or inflammation of the placental membranes that is frequently associated with preterm births and stillbirths [9]. Globally, preterm birth is a significant contributor to neonatal death. Every year, approximately 6,000,000 births are preterm and more than 500,000 neonates die due to prematurity, accounting for 44% of all under-five deaths [10, 11]. The majority of early preterm births are due to microbial infection [9], and approximately 10% are attributable to GBS [12–14]. The bacterial and host determinants that promote GBS vaginal colonization, ascending infection and adverse perinatal outcomes are poorly understood.
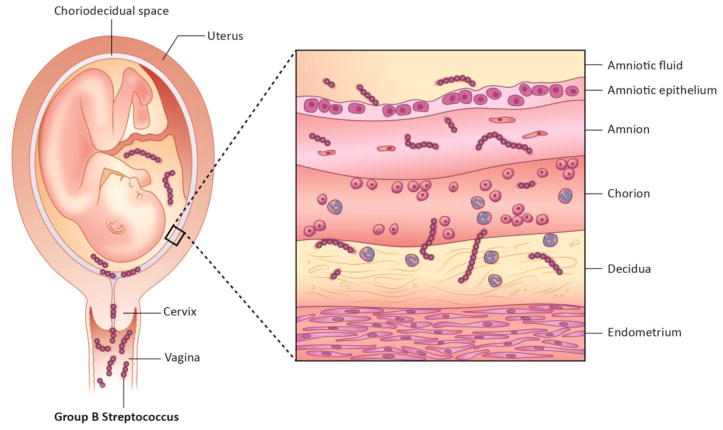
Figure 1. Ascending GBS infection.
GBS vaginal colonization increases the risk of ascending infection during pregnancy. Ascending GBS infection during pregnancy involves bacterial trafficking from the vagina ultimately leading to bacterial invasion of placental membranes (chorion and amnion), the amniotic cavity and fetus.
GBS express a number of virulence factors that promote vaginal colonization, adhesion and invasion of host cells, activation of inflammatory responses or conversely for suppression of inflammatory responses (see Table 1 and Figure 2). These factors increase the risk of ascending infection, fetal injury or preterm birth.
Recently, significant effort has been dedicated to measuring the global rates of GBS colonization (Box 1) [15, 16], invasive disease [8, 17, 18], and related risk factors [19, 20]. These show that increased GBS colonization in many low-income countries correlates with increased neonatal infection and preterm birth [11]. Additionally, women of African descent, have a higher incidence of GBS vaginal colonization [15, 21, 22] and neonatal disease [23–25]. In the U.S. and many other countries, women are routinely screened in the late third trimester (between 35–37 weeks gestation) for GBS colonization by rectovaginal swab and subsequent culture [26]. If the rectovaginal swab is culture positive or if the patient has GBS in the urine, or has a prior history of GBS perinatal infection, intrapartum prophylactic antibiotics are administered to prevent vertical transmission of GBS to the neonate during labor and delivery. Unlike the U.S., some countries have not adopted the GBS screening program but instead administer antibiotics upon the development of a risk factor for GBS neonatal disease (e.g. prolonged rupture of membranes) [26]. However, these approaches have not fully eliminated neonatal GBS infections. This is because these prevention strategies do not address the risk of ascending infection, which can potentially occur anytime during pregnancy leading to preterm birth or stillbirth. Also, these approaches do not prevent late onset GBS infections (observed in neonates who are older than one week of age) where vertical transmission is not the only mode of acquisition [27]. Overall, prevention of GBS infection in pregnancy is still a complex question with risk likely imparted by several factors including: pathogenicity of the GBS strain, host factors, influence of the vaginal/rectal microbiome, false negative screening results, and/or changes in GBS antibiotic resistance. As current interventions targeting GBS infections are limited to antibiotic therapy, and given that antibiotic resistance is on the rise [28], a deeper understanding of how GBS are able to colonize the vagina and cause neonatal disease is critical for the development of new therapeutics. Recently, a number of studies have described host and bacterial factors important for GBS infections during pregnancy. In this review, we discuss recent advancements in GBS pregnancy associated infections and therapeutic strategies.
Box 1. Limited Knowledge of Colonization Prevalence and Risk.
While regional GBS vaginal colonization rates have been estimated [1], colonization rates in many individual countries are unknown. Estimates in countries of GBS vaginal colonization can be highly variable, with inter-study rates differing by as much as 20% [1]. This variability may be due to differences between sub-regions of large countries (e.g. India) or the means of diagnosing colonization (culture-based methods vs. PCR-based methods vs. serology-based methods). More comprehensive country-based and sub-regional-based studies are necessary to fully understand the burden of GBS colonization. New studies should focus on areas where preterm birth rates and neonatal mortality rates are especially high, such as Sub-Saharan Africa or south Asia. Additionally, few studies provide information about GBS serotype prevalence, colonization load, antibiotic resistance profiles, or valuable genetic information, such as virulence gene prevalence. While diagnostic technologies exist to evaluate these indicators, they can be time-consuming, expensive, technically challenging, and overall impractical. Diagnostic methodology for GBS colonization has not advanced as rapidly as our understanding of the disease itself, and new technologies need to be developed to garner more information from future studies to further refine our knowledge of GBS colonization. Finally, more studies need to be designed to conclusively identify risk factors for GBS colonization, ascending infection, and GBS-associated preterm birth. It is clear that previous GBS colonization is a risk factor for colonization during a subsequent pregnancy [2], but few risk factors for initial GBS colonization have been identified. Recent studies have identified obesity [3, 4] and black ethnicity as possible risk factors for colonization [9]. Future studies should be designed to identify population characteristics beyond ethnic demography and age of GBS colonized women in an effort to improve our ability to identify at-risk individuals. Ultimately, universal screening programs are needed in more countries to measure the burden of GBS colonization and successfully prevent disease.
Mechanisms for GBS Infection During Pregnancy
It is long known that GBS vaginal colonization during pregnancy is associated with increased rates of neonatal infection [29], recurrent maternal colonization [30], early term birth (weeks 37 to 38 and 6 days of gestation) [31], preterm birth (weeks 14 to 36 and 6 days of gestation) [3], and stillbirth [32]. GBS is thought to be transmitted from person-to-person via multiple routes including fecal-oral, sexual, and vertical transmission [33]. In the same woman, the close proximity of the vagina and rectum likely enables GBS trafficking from intestinal flora into the vagina. Once GBS enters the vagina, colonization requires the bacteria to overcome a number of challenges, including: physical barriers created by the mucus and epithelial layers, low environmental pH, antimicrobial peptides, antibodies, microbicidal immune cells and a vaginal microbiome dominated by lactobacilli. How a non-motile bacterium, such as GBS, manages to ascend into the uterus while evading host responses is not completely understood. The process of ascending infection is challenging to study, as in vitro models are unsuitable, and in vivo models are limited in their ability to recapitulate human pregnancy [34, 35]. Despite these limitations, a number of animal models, including pregnant mice and nonhuman primates, have been developed to study the mechanisms of ascending GBS infection [36–42], shedding new light on these complicated processes. While studies using these models have revealed novel insight into the role of virulence factors that contribute to ascending infection, more research is needed to fully understand the process of ascending GBS infection and adverse neonatal outcomes.
The host immune response evoked in the placenta in response to GBS infection is a key determinant of perinatal outcome, microbial invasion of the amniotic cavity (MIAC) and fetal injury. A variety of fetal and maternal cells within the placental membranes are capable of pathogen recognition for initiating and sustaining an inflammatory response; these include amniotic epithelial cells, fetal macrophages, decidual macrophages, decidual NK cells, and neutrophils [9, 39, 43–46]. While a severe infection leading to early preterm birth is typically associated with MIAC, an inflammatory response confined to the placenta even in the absence of MIAC is also sufficient to induce preterm labor in some cases [38]. Interestingly, intra-amniotic administration of cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) alone (i.e. without any bacteria) can induce preterm labor in pregnant nonhuman primates [47], and interleukin-1 alpha (IL-1α), IL-1β, interleukin-6 (IL-6), and interleukin-8 (IL-8) drive infection-associated preterm birth in humans (reviewed in [48] & [9]). Thus, placental inflammation induced by bacterial infection is likely a critical component of infection-associated preterm birth. Also, bacterial suppression of placental immune responses could contribute to MIAC leading to stillbirths. A better understanding of the mechanisms by which in utero GBS infections drive preterm births or stillbirths may lead to development of new interventions to reduce the burden of disease. Below, we describe key bacterial and host factors that have been identified to influence GBS colonization and perinatal infection.
Bacterial Factors that Promote GBS Vaginal Colonization, Ascending Infection, and Preterm Birth
Adherence and Invasion Factors
GBS encodes a number of virulence factors that allow it to persist in the harsh vaginal environment and avoid clearance (Table 1). Many of these factors are involved in adherence to and invasion of host epithelial cells that enable persistent colonization [49]. Adherence and invasion appears to be mediated by GBS interactions with host extracellular matrix components (ECM); these interactions may also promote GBS resistance to mechanical clearance, avoidance of immune surveillance and paracellular transmigration [50]. A few examples of GBS interaction with host ECM are discussed below. The GBS extracellular protein BsaB (bacterial surface adhesin of GBS, also known as FbsC) interacts with host laminin [51] and fibrinogen [52], leading to increased adherence to cervicovaginal epithelial cells and biofilm formation [51, 52]. The GBS Srr (serine-rich repeat) family of glycoproteins binds to epithelial cells [53] and interacts with host fibrinogen through a unique “dock, lock, and latch” mechanism [54]. Fibrinogen binding leads to an ordered series of conformational changes in Srr1 and Srr2 that results in enhanced adherence [54]. Deletion of the entire Srr1 glycoprotein or only the latch domain of Srr1 decreases vaginal colonization [55, 56]. The GBS pili also mediate adherence during vaginal colonization via the binding of the PilA adhesin to host cell molecules. However, there is a discrepancy on the nature of the host cell molecules wherein some studies indicated that the PilA adhesion binds collagen type 1 [55, 57] but others indicated that GBS clinical isolates do not bind collagen type 1 but rather bind to fibrinogen [58]. Further studies will provide more insight into these factors during in vivo GBS colonization and infection. Finally, the GBS Alpha C protein, which contains a glycosaminoglycan binding domain is thought to mediate GBS invasion of cervical epithelial cells [59, 60], however, the specific glycosaminoglycan that binds Alpha C is not known. GBS invasion in endometrial cells is mediated via lipid rafts and signaling through phosphoinositide 3-kinase [61]. While clinical GBS isolates have been shown to be able to interact with ECM molecules [56, 58] (Table 1), interactions between GBS and host ECM components during human vaginal colonization are unknown. A better understanding of factors that regulate GBS vaginal colonization is necessary for the development of preventive therapies. Given that there is no known benefit for humans to be vaginally colonized by GBS, elimination of colonization during pregnancy is ideal for the prevention of GBS disease.
Table 1.
GBS Factors Involved in Vaginal Colonization, Ascending Infection or Preterm Birth
| Virulence Factor | Host Target | Function | Phenotype/Models | Reference |
|---|---|---|---|---|
| HylB | Hyaluronic Acid | Blocks TLR2/4 signaling/ | Mouse Vaginal Colonization and Ascending Infection | [37, 42] |
| BsaB/FbsC | Fibrinogen and laminin | Adherence to vaginal epithelial cells | Immortalized human cell line | [51, 52] |
| Hemolytic Pigment | Amnion Epithelial cells Neutrophils Mast cells Macrophages |
Cytolysis Resistance to Neutrophils Mast cell degranulation Pyroptosis |
Mouse Vaginal Colonization, Human choriomamnion/placenta, Nonhuman primate model Primary human cells Immortalized human cell line/s |
[36, 39, 46, 62, 67, 69, 103] |
| Srr1/Srr2 | Fibrinogen | Adherence to vaginal epithelial cells, cervical epithelial cells | Mouse Vaginal Colonization, Immortalized human cell line | [53, 55, 56, 134] |
| Pili | Collagen I | Adherence to vaginal epithelial cells | Mouse Vaginal Colonization, Immortalized human cell line | [55] |
| Capsule | Siglecs | Adherence to and invasion of cervical epithelial cells Blunts siglec signaling in amnion epithelial cells and neutrophils |
Immortalized human cell line Primary human cells |
[50] [98] |
| Alpha C Protein | Host cell surface glycosaminoglycan | Invasion of cervical epithelial cells | Immortalized human cell line | [59] |
| Two Component System | External Signal | Function | Model | Reference |
| CovR/S | pH | Regulates hemolytic pigment expression (apart from other factors), Adherence to vaginal epithelial cells and cervical epithelial cells | Mouse Vaginal Colonization, Immortalized human cell line | [68, 69, 72] |
| FspS/R | fructose 6-phosphate | Vaginal persistence | Mouse Vaginal Colonization | [135] |
Hemolytic Pigment
GBS are β-hemolytic bacteria and the hemolytic property of GBS is important for infection and immune evasion. Hemolytic activity of GBS is due to the ornithine rhamnolipid pigment (hereafter referred to as “hemolytic pigment” or “pigment”) [62], which is produced by the genes of the cyl operon [63]. Transcription of cyl genes and therefore the hemolytic pigment is negatively regulated by the CovR/S two component system (also known as CsrR/S) [62, 64, 65]. Consequently, deletion of covR/S renders GBS hyper-hemolytic and hyper-pigmented [62, 64, 65]. Conversely, deletion of the cylE gene, which encodes an N-acyl transferase necessary for pigment production [62], renders GBS non-pigmented and non-hemolytic [62, 63]. Identification of hemolytic, hyper-hemolytic and non-hemolytic GBS strains in human cases allowed for a greater understanding of the role of hemolysin to GBS infection.
Whidbey et al. described that the hemolytic pigment promoted GBS penetration of human placenta (chorioamniotic membranes) and induced loss of barrier function in human amniotic epithelial cells [62]. Furthermore, hyper-pigmented GBS strains were isolated from either the amniotic fluid or chorioamniotic membranes of women in preterm labor [62]. Randis et al. also noted decreased bacterial dissemination, fetal injury and preterm birth in mice that were vaginally inoculated with non-hemolytic GBS (i.e. GBS lacking cylE) [36]. Recently, Boldenow et al. showed that the increased hemolytic pigment expression accelerated GBS invasion of the amniotic cavity with significant uterine contractions and inflammatory responses indicative of preterm labor in a nonhuman primate model [39]. Although infection with hyper-pigmented GBS induced the formation of neutrophil extracellular traps (NETs) in chorioamniotic membranes of nonhuman primates [39], these GBS strains were resistant to the antimicrobial activity of NETs likely through increased pigment-mediated antioxidant activity [66]. Formation of NETs in response to GBS infection was also observed in murine models of colonization [67] and ascending infection [41]. Collectively, these studies indicate a role for the hemolytic pigment in promoting GBS dissemination in uterine, placental and fetal tissues during pregnancy.
The GBS hemolytic pigment also affects vaginal colonization. Absence of the hemolytic pigment reduced the ability of GBS to successfully colonize the vagina, possibly due to increased susceptibility to neutrophil clearance [36, 67]. Surprisingly, hyper-pigmented GBS also exhibited decreased vaginal colonization in mice ([68, 69]; see [70] for visual of murine vaginal model), likely due to increased pigment mediated stimulation of neutrophil [68] and mast cell [69] inflammatory pathways. Consistent with these observations, hyper-pigmented GBS were rarely isolated from rectovaginal swabs of asymptomatic pregnant women [69]. These results emphasize the role of vaginal immune responses in pathogen colonization.
Apart from immune cells, pH regulates GBS gene expression and therefore influences vaginal colonization. For instance, the GBS CovR/S system responds to pH wherein increased CovR/S regulation was observed under low (acidic) pH [65, 71–73]. Changes in pH also influences GBS adhesion [72, 74], survival [75], and biofilm formation [76, 77]. Of note, high vaginal pH and a non-lactobacilli dominated vaginal microbiome [78] are associated with women of African descent with higher incidence of GBS vaginal colonization [15, 21, 22] and neonatal disease [23–25]. These studies indicate that GBS responds to environmental cues such as pH and even utilizes regulatory systems such as CovR/S to temporally control virulence factor expression (e.g. hemolytic pigment) during pregnancy-associated infections. As such, this makes the GBS pigment an intriguing target for vaccine development.
Hyaluronidase
The GBS hyaluronidase, known as HylB, promotes vaginal colonization [42]. HylB is secreted by GBS and specifically targets and degrades host hyaluronic acid [79, 80]. Hyaluronic acid is an extracellular matrix glycosaminoglycan composed of repeating disaccharide units (N-Acetyl-D-Glucosamine-D-Glucuronic acid) and is important for cell migration, cell signaling, regulation of inflammation [81] and prevention of ascending infection [82, 83]. GBS HylB degrades host hyaluronic acid into its disaccharide components, which are immunosuppressive as they bind to TLR2/TLR4 receptors and block signaling [42]. Deletion of hylB led to increased clearance of GBS from the mouse vagina [42]. Similarly, GBS lacking HylB were less able to ascend from the vagina to the uterus and were diminished for their ability to invade fetal tissues and cause preterm birth [37]. In contrast, uterine tissue infected with HylB proficient GBS showed decreased levels of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), IL-6, and IL-8, leading to bacterial ascension [37]. Thus, suppression of key inflammatory responses also plays an important role in GBS infection associated fetal injury.
Other Virulence Factors
Recent studies have described a role for extracellular membrane vesicles (MVs) in weakening of placental membranes [84]. GBS MVs contained multiple virulence factors, including: 1) HylB, 2) CAMP factor (Christine, Atkins, Munch-Peterson factor [85]), a secreted pore-forming protein [86] that may amplify [87], but is not essential for GBS virulence [88], 3) IgA binding protein, with the ability to bind human IgA [89] for host immune evasion [90] and 4) multiple enzymes that may regulate ECM degradation [84]. Intra-amniotic administration of GBS MVs in pregnant mice caused significant damage to choriodecidual tissues and stimulated leukocytic infiltration and inflammation, leading to membrane weakening [84]. The specific role played by each virulence factors in the context of MVs remains unknown. MV weakening of choriodecidual membranes represents a novel mechanism of GBS fetal injury.
Host Determinants of GBS Vaginal Colonization, Ascending Infection, and Preterm Birth
Vaginal Colonization
Evasion of the host immune response is essential for successful vaginal colonization. GBS vaginal immunity is mediated by its ability to resist many physical and cellular barriers including the luminal mucus layer, vaginal epithelia, and immune cells in the vagina. Recent work using animal models have provided new information regarding the host immune response to GBS vaginal colonization [49, 67–69]. Vaginal immune responses to GBS are largely mediated by neutrophils [49, 67, 68], mast cells [69], and macrophages [67] (Figure 2). The role of NK cells and dendritic cells in GBS colonization is not known. Multiple soluble inflammatory cytokines and chemokines have been identified as important for reducing GBS vaginal colonization and include IL-1β, IL-6, IL-8, IL-17, IL-23, and histamine [49, 67, 68]. Currently, the mucosal T cell response to GBS colonization is ill-defined. Studies have shown that IL-17 and IL-17+ cells play an important role in clearance of a hyper-adherent and invasive GBS strain from the vagina [49], suggesting that the Th17 differentiation pathway is important for controlling persistent GBS colonization. Similarly, another study found cytokines involved in Th1, Th2, and Th17 differentiation pathways as important for decreased colonization [67]; however, T cells were not directly identified as being important in either study.
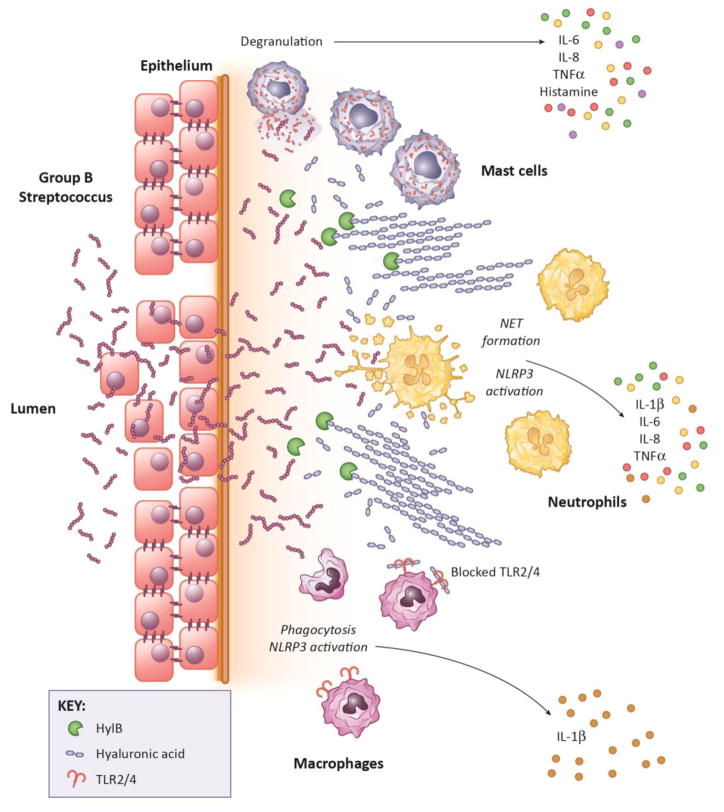
Figure 2. GBS Interaction with Innate Immune Cells During Genital Infection.
Upon primary vaginal colonization, vaginal-resident mast cells are activated and de-granulated through the hemolytic activity of the GBS pigment. De-granulation leads to the release of inflammatory mediators, including IL-6, IL-8, TNFα, and histamine, which in turn recruits other immune cells, such as neutrophils and macrophages, to aid in bacterial clearance [69]. Neutrophils clear GBS through phagocytosis and extrusion of NETs [67]. Additionally, GBS activates the NLRP3 inflammasome in neutrophils, leading to secretion of IL-1β, as well as other inflammatory cytokines. GBS are able to use the pigment to prevent phagocytic death through sequestration of reactive oxygen species [66], and to avoid being killed by NETs [39]. Macrophages also aid in GBS clearance through phagocytosis. GBS, in turn, activate the NLRP3 inflammasome in macrophages through membrane permeabilization by pigment, leading to pyroptosis and fetal damage during pregnancy [46].
Infection of Placental Membranes
Many studies have focused on GBS infection of the placental membranes (chorioamnion) in order to understand how GBS penetrates these barriers and induces chorioamnionitis. GBS are able to adhere to and invade both chorionic and amniotic epithelial cells [62, 91], which are partially mediated by several factors: 1) IagA, a glycosyltransferase that helps anchor lipoteichoic acid to the cell surface [92], 2) the hemolytic pigment and its regulator CovR/S [62], and 3) quorum sensing mediated by genes in the rgf operon [93]. GBS has also been shown to induce secretion of multiple cytokines and defensins from placental membranes ex vivo, including TNF-α, IL-1α, IL-1β, IL-6, and IL-8 [44, 62, 94–96]. Inflammation is stimulated either through pattern-recognition receptor (PRR) sensing of GBS antigens [44, 94] or by pigment-mediated activation of nuclear factor-κB (NF-κB) [62]. An important family of PRRs that mediates placental membrane immunity are the Siglecs [97, 98], a family of cell-surface sialic acid binding lectins that regulate innate and adaptive immune function [99]. GBS are able to bind Siglecs through the sialic acid capsule or β-protein to suppress immune cell activation [98, 100–102] and placental membrane inflammation [98], potentially leading to increased rates of GBS-associated preterm birth and stillbirth.
Fetal injury
GBS invasion of the fetus in utero leads to a variety of adverse outcomes, including tissue damage, inflammation, lung and brain injury, pneumonia, meningitis, sepsis, and fetal death. GBS can invade multiple fetal organs, including the lung, blood, liver, spleen and gastrovascular cavity. Fetal tissue damage has been observed in the presence and absence of bacterial invasion, which may be due to inflammation in the gestational tissues and amniotic fluid. GBS invasion of fetal tissues induces inflammation and fetal death [32, 36, 37, 46], and in the case of the hemolytic pigment, this involved induction of the NLRP3 inflammasome [46]. GBS stimulate the NLRP3 inflammasome in a number of immune cells such as dendritic cells [103], macrophages [46] and neutrophils [104], which contribute to in vivo inflammation.
Interestingly, fetal injury is not entirely dependent on bacterial invasion of fetal tissues. Fetal lung injury can also be caused by GBS-induced chorioamnionitis without bacterial invasion [38, 105]. Also, increases in amniotic fluid cytokines contributes to fetal lung injury [38], and dysregulation of fetal lung development [105] [106]. Moreover, intra-amniotic administration of MVs leads to significantly increased rates of fetal damage and preterm birth [84]. These studies suggest that fetal injury can occur during transient or limited infection, and further emphasizes the importance of developing therapeutics that prevent vaginal colonization and ascending infection.
Limitations and Next Steps
While significant research has been committed to understanding the host and bacterial factors involved in GBS vaginal colonization and ascending infection, further research is needed to fully grasp these complex processes. Additionally, the research that has been completed is not without its limitations. These limitations and next steps for research are discussed below.
Vaginal Colonization
Despite recent advances, there are many limitations in our understanding of GBS vaginal colonization. Importantly, the complete repertoire of GBS virulence factors, host factors, and environmental factors that determine the extent and duration of vaginal colonization remain unknown. This point is key given that the dynamics of colonization during pregnancy are highly variable [107]. In addition, experiments performed in laboratory settings are limited by the use of animal models that do not perfectly model the human vaginal environment. To fully understand GBS vaginal colonization, experimental models and outputs that more closely represent human disease are needed. Finally, it is often assumed that neonates contract GBS from contaminated vaginal fluids during the birthing process; however, given the kinetics of neonatal disease (frequently displaying clinical symptoms within 0–24 hours of birth [108]), many cases are thought to be a result of in utero infections caused by ascended GBS. This idea is also supported by the frequent recovery of GBS from the amniotic fluid and chorioamniotic membranes in cases of infection-associated preterm birth [1, 109, 110]. Given its important implication for understanding GBS disease, colonization is an area of active research.
Ascension from the Vagina to the Uterus
While studies have provided some insight into virulence factors that enable GBS to gain access to the uterine space from the lower genital tract, and host factors involved in this process, significant research is required to fully understand ascending GBS infection. Currently, little is known about host factors that prevent ascending infection, such as the role of the cervical barrier, endocrine signaling and cellular immunity. Further, host specific factors and genetics that may influence ascending GBS infection are unknown. Studies aimed at filling these knowledge gaps could lead to the development of therapeutics for preventing ascending infection. The choice of animal model is key in answering some of these questions due to important differences in pregnancy physiology, mechanism of labor and placental structure between humans and mice [111]. Nonhuman primates are the closest animal model to fully recapitulate important aspects of human pregnancy [111] but are limited in their use by ethical constraints, availability and cost. Ideally, a combination of lower animal and nonhuman primate models should be used in order to delineate relevant aspects of disease. Finally, clinical studies should be designed to identify biomarkers for ascending infection. Viral infection of the cervix [112] and diminished cervical hyaluronic acid levels [83] have been associated with increased ascending infection, however, little is known about the clinical relevance of these or other factors in the context of ascending GBS infection.
GBS Prevention and Therapeutics
Currently, strategies focus on the prevention of GBS transmission during labor and delivery through the use of antibiotics. This strategy does not fully capture the biology of GBS infection, nor does it completely address the full burden of GBS disease. Moreover, antibiotic resistance is increasing [28], and use of antibiotics during pregnancy has consequential effects for neonatal health that are only now being appreciated [113, 114]. To successfully eradicate the burden of disease, interventions need to be specifically targeted, have minimal detrimental effects on the microbiome and target processes upstream of vertical transmission, such as colonization and ascending infection.
Multiple studies have focused on a probiotic approach to reducing vaginal GBS colonization. Recent studies using probiotic Lactobacillus species have shown that pretreatment of the vagina prior to GBS colonization can block GBS adherence to vaginal epithelial cells [115], and reduce colonization [116, 117]. Probiotic administration of the oral colonizer Streptococcus salivarius has also been shown to have the ability to reduce vaginal GBS burden through a yet unidentified anti-microbial activity [118]. It is important to note that frequent doses (3–7) of probiotic bacteria were required for a protective effect in vivo [116, 117], raising the question as to the feasibility of probiotic intervention in humans, and highlighting the need to continue to explore this field to identify an efficient and feasible probiotic therapy to prevent GBS colonization. Moreover, little is known about the interactions between GBS and the vaginal microbiome, and whether those interactions have any positive benefits to human health. Further studies that aim to understand these interactions would shed much needed light on this topic.
A vaccine to prevent GBS colonization would be the most effective intervention; however, development of a vaccine has proven to be challenging. Recent work has shown that vaccination of mice with killed bacteria reduces preterm birth rates [119] and that mucosal vaccine delivery is more effective than intramuscular delivery [120]. Multiple studies have identified maternal antibody levels to the GBS capsule as being protective against GBS infection [121–124], and it has long been known that anti-capsular antibodies can confer protection to infection [5]. Unfortunately, vaccines targeting the GBS capsule alone are ineffective due to their poor immunogenicity and thus, conjugate-capsule vaccines and a vaccine that targets the alpha C/Rib protein family are now being tested in clinical trials (Reviewed in [125], [126] & [127]). Despite the promise of these vaccine candidates, challenges to the eradication of GBS disease will exist even after the implementation of a vaccine. While ten GBS capsular serotypes have been identified, serotypes Ia, Ib, II, III, and V are predominantly responsible for GBS disease [127]. Consequently, current vaccine candidates target serotypes Ia, Ib, and III, [125] and a pentavalent vaccine targeting serotypes Ia, Ib, II, III, and V is in pre-clinical development [126]. The development of vaccines targeting various antigens such as Alpha C/Rib may prove to be vital, as non-typeable GBS strains can cause disease [128]. Additionally, GBS strains can switch capsular serotypes [129], thereby evading host immunity conferred by vaccination.
It is possible that if these vaccines are developed, serotypes or strains that are not typically a significant cause of GBS disease may emerge in vaccinated populations. Indeed, this phenomenon has been observed with implementation of conjugate vaccines against Streptococcus pneumoniae [130, 131]. Thus, GBS surveillance programs would need to remain vigilant even after a vaccine becomes widely used. There are also significant challenges in resource-limited settings to consider prior to implementation (Reviewed in [132]). Regardless, it is clear that a vaccine would be the most effective means of reducing the global GBS burden of disease [133].
Concluding Remarks
GBS has long been recognized as a significant human pathogen [6], yet we are only now, beginning to fully understand its pathogenesis almost a century later. It is clear that understanding the interplay between the host and bacteria is vital for understanding how to effectively prevent GBS disease while having minimal adverse effects on the human host. Research in this field has revealed novel insights into the bacterial virulence factors necessary for successfully establishing disease (Table 1) and an appropriate host response to prevent ascending infection and preterm birth (Figures 1, 2). Any disturbance in this balance can lead to serious and lasting outcomes for the host, or disadvantageous pressures for the bacteria. Several examples of how this balance can be perturbed to benefit human health have described above, but more research is needed to fully understand what triggers ascending GBS infection, the host immune response to colonization and ascending infection, and the physiological drivers of fetal damage and preterm birth (see Outstanding Questions). Additionally, more epidemiological data are required to describe the global burden of GBS colonization and disease (Box 1). As GBS colonization during pregnancy is intermittent and variable [107], the current screening methodology likely misses a significant portion of colonization during pregnancy. This issue results in a measurable risk for ascending GBS infection during pregnancy that could lead to stillbirth, preterm birth, and early term birth. An evaluation of earlier and more frequent GBS screening during pregnancy may shed light on these issues. With these data, effective and feasible interventions to prevent GBS disease can be developed. Ultimately, vaccination will prove to be the most effect intervention. A combination of rational vaccine design, intelligent implementation and monitoring strategies, and strong advocacy [26] may lead to the eradication of GBS as a human pathogen.
Outstanding Questions.
Why are humans intermittently colonized with GBS? What determines whether a GBS strain will colonize the vagina? How can we eliminate GBS without adverse consequences on beneficial microbiota?
What factors determine the ability of a GBS strain to ascend into the uterus and invade the placenta and amniotic cavity? Is it environmental factors in the lower genital tract during pregnancy?
How do geographic differences in host genetics, environment and diet contribute to differences in the vaginal microbiome and GBS colonization?
Can we determine which GBS strains are likely to invade the amniotic cavity or cause late-onset neonatal sepsis to better target antibiotic therapy to a higher risk group of pregnant women?
Trends Box.
GBS has to evade genitourinary immune responses for successful colonization and infection
A number of virulence factors promote GBS vaginal colonization and subsequent ascension into the pregnant uterus.
The GBS hemolytic pigment and hyaluronidase enable the pathogen to resist immune responses; however, dysregulation of the hemolytic pigment can also lead to bacterial clearance from the vagina.
GBS invasion of the amniotic fluid is not absolutely necessary for induction of inflammatory responses and preterm birth
Development of a GBS vaccine is critical to reduce or prevent the risk of infection during pregnancy thereby reducing GBS disease burden.
Acknowledgments
We gratefully acknowledge Jan Hamanishi for assistance with graphical design. We apologize to GBS researchers whose work was not reviewed here due to constraints in space and/or focus of the topic. We acknowledge funding from the National Institutes of Health, Grant R01AI100989 to L.R and K. A. W and R21AI125907, and R01AI112619 to L.R. J. V was supported by the NIH training grant T32 AI07509 (PI: Lee Ann Campbell). The content in this manuscript is the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hillier SL, et al. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol. 1991;165(4 Pt 1):955–61. doi: 10.1016/0002-9378(91)90447-y. [DOI] [PubMed] [Google Scholar]
- 2.Allen U, et al. Relationship between antenatal group B streptococcal vaginal colonization and premature labour. Paediatr Child Health. 1999;4(7):465–9. doi: 10.1093/pch/4.7.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn JE, et al. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth. 2010;10(Suppl 1):S1. doi: 10.1186/1471-2393-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norcard N, Mollereau R. Sur une mammite contagieuse des vaches laitieres. Ann Inst Pasteur. 1887;1:109–126. [Google Scholar]
- 5.Lancefield RC, Hare R. The Serological Differentiation of Pathogenic and Non-Pathogenic Strains of Hemolytic Streptococci from Parturient Women. J Exp Med. 1935;61(3):335–49. doi: 10.1084/jem.61.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fry RM. Fatal infections by hemolytic Streptococcus group B. Lancet. 1938;1:199–201. [Google Scholar]
- 7.Le Doare K, Heath PT. An overview of global GBS epidemiology. Vaccine. 2013;31(Suppl 4):D7–12. doi: 10.1016/j.vaccine.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Stoll BJ, et al. Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics. 2011;127(5):817–26. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–5. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–40. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 11.Mokdad AH, et al. Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10036):2383–401. doi: 10.1016/S0140-6736(16)00648-6. [DOI] [PubMed] [Google Scholar]
- 12.Hitti J, et al. Amniotic fluid infection, cytokines, and adverse outcome among infants at 34 weeks’ gestation or less. Obstet Gynecol. 2001;98(6):1080–8. doi: 10.1016/s0029-7844(01)01567-8. [DOI] [PubMed] [Google Scholar]
- 13.DiGiulio DB, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3(8):e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han YW, et al. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47(1):38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwatra G, et al. Prevalence of maternal colonisation with group B Streptococcus: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(9):1076–84. doi: 10.1016/S1473-3099(16)30055-X. [DOI] [PubMed] [Google Scholar]
- 16.Barcaite E, et al. Prevalence of maternal group B streptococcal colonisation in European countries. Acta Obstet Gynecol Scand. 2008;87(3):260–71. doi: 10.1080/00016340801908759. [DOI] [PubMed] [Google Scholar]
- 17.Nan C, et al. Maternal group B Streptococcus-related stillbirth: a systematic review. BJOG. 2015;122(11):1437–45. doi: 10.1111/1471-0528.13527. [DOI] [PubMed] [Google Scholar]
- 18.Petersen KB, et al. Increasing prevalence of group B streptococcal infection among pregnant women. Dan Med J. 2014;61(9):A4908. [PubMed] [Google Scholar]
- 19.Kleweis SM, et al. Maternal Obesity and Rectovaginal Group B Streptococcus Colonization at Term. Infect Dis Obstet Gynecol. 2015;2015:586767. doi: 10.1155/2015/586767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colicchia LC, et al. Recurrence of group B Streptococcus colonization in successive pregnancies. J Perinatol. 2015;35(3):173–6. doi: 10.1038/jp.2014.185. [DOI] [PubMed] [Google Scholar]
- 21.Hickman ME, et al. Changing epidemiology of group B streptococcal colonization. Pediatrics. 1999;104(2 Pt 1):203–9. doi: 10.1542/peds.104.2.203. [DOI] [PubMed] [Google Scholar]
- 22.Taylor JK, Hall RW, Dupre AR. The incidence of group B Streptococcus in the vaginal tracts of pregnant women in central Alabama. Clin Lab Sci. 2002;15(1):16–7. [PubMed] [Google Scholar]
- 23.Lin FY, et al. Prematurity is the major risk factor for late-onset group B Streptococcus disease. J Infect Dis. 2003;188(2):267–71. doi: 10.1086/376457. [DOI] [PubMed] [Google Scholar]
- 24.Peltier MR, et al. Amniotic fluid and maternal race influence responsiveness of fetal membranes to bacteria. J Reprod Immunol. 2012;96(1–2):68–78. doi: 10.1016/j.jri.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weston EJ, et al. The burden of invasive early-onset neonatal sepsis in the United States 2005–2008. Pediatr Infect Dis J. 2011;30(11):937–41. doi: 10.1097/INF.0b013e318223bad2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns G, Plumb J. GBS public awareness, advocacy, and prevention--what’s working, what’s not and why we need a maternal GBS vaccine. Vaccine. 2013;31(Suppl 4):D58–65. doi: 10.1016/j.vaccine.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 27.Tazi A, et al. The surface protein HvgA mediates group B Streptococcus hypervirulence and meningeal tropism in neonates. J Exp Med. 2010;207(11):2313–22. doi: 10.1084/jem.20092594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castor ML, et al. Antibiotic resistance patterns in invasive group B streptococcal isolates. Infect Dis Obstet Gynecol. 2008;2008:727505. doi: 10.1155/2008/727505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoogkamp-Korstanje JA, Gerards LJ, Cats BP. Maternal carriage and neonatal acquisition of group B streptococci. J Infect Dis. 1982;145(6):800–3. doi: 10.1093/infdis/145.6.800. [DOI] [PubMed] [Google Scholar]
- 30.Cheng PJ, et al. Risk factors for recurrence of group B Streptococcus colonization in a subsequent pregnancy. Obstet Gynecol. 2008;111(3):704–9. doi: 10.1097/AOG.0b013e318163cd6b. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell K, et al. Group B Streptococcus colonization and higher maternal IL-1beta concentrations are associated with early term births. J Matern Fetal Neonatal Med. 2013;26(1):56–61. doi: 10.3109/14767058.2012.725789. [DOI] [PubMed] [Google Scholar]
- 32.Monari F, et al. Fetal bacterial infections in antepartum stillbirth: a case series. Early Hum Dev. 2013;89(12):1049–54. doi: 10.1016/j.earlhumdev.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Manning SD, et al. Prevalence of group B Streptococcus colonization and potential for transmission by casual contact in healthy young men and women. Clin Infect Dis. 2004;39(3):380–8. doi: 10.1086/422321. [DOI] [PubMed] [Google Scholar]
- 34.De Clercq E, Kalmar I, Vanrompay D. Animal models for studying female genital tract infection with Chlamydia trachomatis. Infect Immun. 2013;81(9):3060–7. doi: 10.1128/IAI.00357-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDuffie RS, Gibbs RS. Animal models of ascending genital-tract infection in pregnancy. Infect Dis Obstet Gynecol. 1994;2(2):60–70. doi: 10.1155/S1064744994000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Randis TM, et al. Group B Streptococcus beta-hemolysin/cytolysin breaches maternal-fetal barriers to cause preterm birth and intrauterine fetal demise in vivo. J Infect Dis. 2014;210(2):265–73. doi: 10.1093/infdis/jiu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vornhagen J, et al. Bacterial Hyaluronidase Promotes Ascending GBS Infection and Preterm Birth. MBio. 2016;7(3) doi: 10.1128/mBio.00781-16. pii: e00781-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams Waldorf KM, et al. Choriodecidual group B streptococcal inoculation induces fetal lung injury without intra-amniotic infection and preterm labor in Macaca nemestrina. PLoS One. 2011;6(12):e28972. doi: 10.1371/journal.pone.0028972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boldenow E, et al. Group B Streptococcus Circumvents Neutrophils and Neutrophil Extracellular Traps during Amniotic Cavity Invasion and Preterm Labor. Sci Immunol. 2016;1(4):eaah4576. doi: 10.1126/sciimmunol.aah4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ancona RJ, Ferrieri P. Experimental vaginal colonization and mother-infant transmission of group B streptococci in rats. Infect Immun. 1979;26(2):599–603. doi: 10.1128/iai.26.2.599-603.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kothary V, et al. Group B Streptococcus Induces Neutrophil Recruitment to Gestational Tissues and Elaboration of Extracellular Traps and Nutritional Immunity. Frontiers in Cellular and Infection Microbiology. 2017;7(19) doi: 10.3389/fcimb.2017.00019. p. fcimb.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolar SL, et al. Group B Streptococcus Evades Host Immunity by Degrading Hyaluronan. Cell Host Microbe. 2015;18(6):694–704. doi: 10.1016/j.chom.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh U, et al. Immunological properties of human decidual macrophages--a possible role in intrauterine immunity. Reproduction. 2005;129(5):631–7. doi: 10.1530/rep.1.00331. [DOI] [PubMed] [Google Scholar]
- 44.Boldenow E, et al. Role of cytokine signaling in group B Streptococcus-stimulated expression of human beta defensin-2 in human extraplacental membranes. Am J Reprod Immunol. 2015;73(3):263–72. doi: 10.1111/aji.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duriez M, et al. Human decidual macrophages and NK cells differentially express Toll-like receptors and display distinct cytokine profiles upon TLR stimulation. Front Microbiol. 2014;5:316. doi: 10.3389/fmicb.2014.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whidbey C, et al. A streptococcal lipid toxin induces membrane permeabilization and pyroptosis leading to fetal injury. EMBO Mol Med. 2015;7(4):488–505. doi: 10.15252/emmm.201404883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadowsky DW, et al. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195(6):1578–89. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 48.Cappelletti M, et al. Inflammation and preterm birth. J Leukoc Biol. 2016;99(1):67–78. doi: 10.1189/jlb.3MR0615-272RR. [DOI] [PubMed] [Google Scholar]
- 49.Patras KA, et al. Characterization of host immunity during persistent vaginal colonization by Group B Streptococcus. Mucosal Immunol. 2015;8(6):1339–48. doi: 10.1038/mi.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soriani M, et al. Group B Streptococcus crosses human epithelial cells by a paracellular route. J Infect Dis. 2006;193(2):241–50. doi: 10.1086/498982. [DOI] [PubMed] [Google Scholar]
- 51.Jiang S, Wessels MR. BsaB, a novel adherence factor of group B Streptococcus. Infect Immun. 2014;82(3):1007–16. doi: 10.1128/IAI.01014-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buscetta M, et al. FbsC, a novel fibrinogen-binding protein, promotes Streptococcus agalactiae-host cell interactions. J Biol Chem. 2014;289(30):21003–21015. doi: 10.1074/jbc.M114.553073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mistou MY, et al. Molecular dissection of the secA2 locus of group B Streptococcus reveals that glycosylation of the Srr1 LPXTG protein is required for full virulence. J Bacteriol. 2009;191(13):4195–206. doi: 10.1128/JB.01673-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seo HS, et al. Characterization of fibrinogen binding by glycoproteins Srr1 and Srr2 of Streptococcus agalactiae. J Biol Chem. 2013;288(50):35982–96. doi: 10.1074/jbc.M113.513358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheen TR, et al. Serine-rich repeat proteins and pili promote Streptococcus agalactiae colonization of the vaginal tract. J Bacteriol. 2011;193(24):6834–42. doi: 10.1128/JB.00094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang NY, et al. Group B streptococcal serine-rich repeat proteins promote interaction with fibrinogen and vaginal colonization. J Infect Dis. 2014;210(6):982–91. doi: 10.1093/infdis/jiu151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banerjee A, et al. Bacterial pili exploit integrin machinery to promote immune activation and efficient blood-brain barrier penetration. Nat Commun. 2011;2:462. doi: 10.1038/ncomms1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dramsi S, et al. Epidemiologically and clinically relevant Group B Streptococcus isolates do not bind collagen but display enhanced binding to human fibrinogen. Microbes Infect. 2012;14(12):1044–8. doi: 10.1016/j.micinf.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Bolduc GR, et al. The alpha C protein mediates internalization of group B Streptococcus within human cervical epithelial cells. Cell Microbiol. 2002;4(11):751–8. doi: 10.1046/j.1462-5822.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- 60.Baron MJ, et al. Identification of a glycosaminoglycan binding region of the alpha C protein that mediates entry of group B streptococci into host cells. J Biol Chem. 2007;282(14):10526–36. doi: 10.1074/jbc.M608279200. [DOI] [PubMed] [Google Scholar]
- 61.Goluszko P, et al. Group B Streptococcus exploits lipid rafts and phosphoinositide 3-kinase/Akt signaling pathway to invade human endometrial cells. Am J Obstet Gynecol. 2008;199(5):548 e1–9. doi: 10.1016/j.ajog.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 62.Whidbey C, et al. A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. J Exp Med. 2013;210(6):1265–81. doi: 10.1084/jem.20122753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pritzlaff CA, et al. Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Mol Microbiol. 2001;39(2):236–47. doi: 10.1046/j.1365-2958.2001.02211.x. [DOI] [PubMed] [Google Scholar]
- 64.Lamy MC, et al. CovS/CovR of group B Streptococcus: a two-component global regulatory system involved in virulence. Mol Microbiol. 2004;54(5):1250–68. doi: 10.1111/j.1365-2958.2004.04365.x. [DOI] [PubMed] [Google Scholar]
- 65.Jiang SM, et al. Regulation of virulence by a two-component system in group B Streptococcus. J Bacteriol. 2005;187(3):1105–13. doi: 10.1128/JB.187.3.1105-1113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu GY, et al. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc Natl Acad Sci U S A. 2004;101(40):14491–6. doi: 10.1073/pnas.0406143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carey AJ, et al. Infection and cellular defense dynamics in a novel 17beta-estradiol murine model of chronic human group B Streptococcus genital tract colonization reveal a role for hemolysin in persistence and neutrophil accumulation. J Immunol. 2014;192(4):1718–31. doi: 10.4049/jimmunol.1202811. [DOI] [PubMed] [Google Scholar]
- 68.Patras KA, et al. Group B Streptococcus CovR regulation modulates host immune signalling pathways to promote vaginal colonization. Cell Microbiol. 2013;15(7):1154–67. doi: 10.1111/cmi.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gendrin C, et al. Mast cell degranulation by a hemolytic lipid toxin decreases GBS colonization and infection. Sci Adv. 2015;1(6):e1400225. doi: 10.1126/sciadv.1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patras KA, Doran KS. A Murine Model of Group B Streptococcus Vaginal Colonization. J Vis Exp. 2016;(117) doi: 10.3791/54708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cumley NJ, et al. The CovS/CovR acid response regulator is required for intracellular survival of group B Streptococcus in macrophages. Infect Immun. 2012;80(5):1650–61. doi: 10.1128/IAI.05443-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park SE, Jiang S, Wessels MR. CsrRS and environmental pH regulate group B Streptococcus adherence to human epithelial cells and extracellular matrix. Infect Immun. 2012;80(11):3975–84. doi: 10.1128/IAI.00699-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santi I, et al. CsrRS regulates group B Streptococcus virulence gene expression in response to environmental pH: a new perspective on vaccine development. J Bacteriol. 2009;191(17):5387–97. doi: 10.1128/JB.00370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tamura GS, et al. Adherence of group B streptococci to cultured epithelial cells: roles of environmental factors and bacterial surface components. Infect Immun. 1994;62(6):2450–8. doi: 10.1128/iai.62.6.2450-2458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borges S, Silva J, Teixeira P. Survival and biofilm formation by Group B streptococci in simulated vaginal fluid at different pHs. Antonie Van Leeuwenhoek. 2012;101(3):677–82. doi: 10.1007/s10482-011-9666-y. [DOI] [PubMed] [Google Scholar]
- 76.D’Urzo N, et al. Acidic pH strongly enhances in vitro biofilm formation by a subset of hypervirulent ST-17 Streptococcus agalactiae strains. Appl Environ Microbiol. 2014;80(7):2176–85. doi: 10.1128/AEM.03627-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ho YR, et al. The enhancement of biofilm formation in Group B streptococcal isolates at vaginal pH. Med Microbiol Immunol. 2013;202(2):105–15. doi: 10.1007/s00430-012-0255-0. [DOI] [PubMed] [Google Scholar]
- 78.Ravel J, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–7. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gochnauer TA, Wilson JB. The production of hyaluronidase by Lancefield’s Group B streptococci. J Bacteriol. 1951;62(4):405–14. doi: 10.1128/jb.62.4.405-414.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baker JR, Pritchard DG. Action pattern and substrate specificity of the hyaluronan lyase from group B streptococci. Biochem J. 2000;348(Pt 2):465–71. [PMC free article] [PubMed] [Google Scholar]
- 81.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85(8):699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 82.Mahendroo M. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction. 2012;143(4):429–38. doi: 10.1530/REP-11-0466. [DOI] [PubMed] [Google Scholar]
- 83.Akgul Y, et al. Hyaluronan in cervical epithelia protects against infection-mediated preterm birth. J Clin Invest. 2014;124(12):5481–9. doi: 10.1172/JCI78765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Surve MV, et al. Membrane Vesicles of Group B Streptococcus Disrupt Feto-Maternal Barrier Leading to Preterm Birth. PLoS Pathog. 2016;12(9):e1005816. doi: 10.1371/journal.ppat.1005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Christie R, Atkins NE, Munch-Petersen E. A note on a lytic phenomenon shown by group B streptococci. Aust J Exp Biol Med Sci. 1944;22:197–200. doi: 10.1038/icb.1945.30. [DOI] [PubMed] [Google Scholar]
- 86.Lang S, Palmer M. Characterization of Streptococcus agalactiae CAMP factor as a pore-forming toxin. J Biol Chem. 2003;278(40):38167–73. doi: 10.1074/jbc.M303544200. [DOI] [PubMed] [Google Scholar]
- 87.Jurgens D, Sterzik B, Fehrenbach FJ. Unspecific binding of group B streptococcal cocytolysin (CAMP factor) to immunoglobulins and its possible role in pathogenicity. J Exp Med. 1987;165(3):720–32. doi: 10.1084/jem.165.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hensler ME, et al. CAMP factor is not essential for systemic virulence of Group B Streptococcus. Microb Pathog. 2008;44(1):84–8. doi: 10.1016/j.micpath.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kvam AI, Iversen OJ, Bevanger L. Binding of human IgA to HCl-extracted c protein from group B streptococci (GBS) APMIS. 1992;100(12):1129–32. doi: 10.1111/j.1699-0463.1992.tb04050.x. [DOI] [PubMed] [Google Scholar]
- 90.Nordstrom T, et al. Human Siglec-5 inhibitory receptor and immunoglobulin A (IgA) have separate binding sites in streptococcal beta protein. J Biol Chem. 2011;286(39):33981–91. doi: 10.1074/jbc.M111.251728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Winram SB, et al. Characterization of group B streptococcal invasion of human chorion and amnion epithelial cells in vitro. Infect Immun. 1998;66(10):4932–41. doi: 10.1128/iai.66.10.4932-4941.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doran KS, et al. Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J Clin Invest. 2005;115(9):2499–507. doi: 10.1172/JCI23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parker RE, et al. Contribution of the RgfD Quorum Sensing Peptide to rgf Regulation and Host Cell Association in Group B Streptococcus. Genes (Basel) 2017;8(1) doi: 10.3390/genes8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boldenow E, et al. The trichloroethylene metabolite S-(1,2-dichlorovinyl)-l-cysteine but not trichloroacetate inhibits pathogen-stimulated TNF-alpha in human extraplacental membranes in vitro. Reprod Toxicol. 2015;52:1–6. doi: 10.1016/j.reprotox.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boldenow E, et al. Antimicrobial peptide response to group B Streptococcus in human extraplacental membranes in culture. Placenta. 2013;34(6):480–5. doi: 10.1016/j.placenta.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flores-Herrera H, et al. An experimental mixed bacterial infection induced differential secretion of proinflammatory cytokines (IL-1beta, TNFalpha) and proMMP-9 in human fetal membranes. Placenta. 2012;33(4):271–7. doi: 10.1016/j.placenta.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 97.Brinkman-Van der Linden EC, et al. Human-specific expression of Siglec-6 in the placenta. Glycobiology. 2007;17(9):922–31. doi: 10.1093/glycob/cwm065. [DOI] [PubMed] [Google Scholar]
- 98.Ali SR, et al. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J Exp Med. 2014;211(6):1231–42. doi: 10.1084/jem.20131853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255–66. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 100.Carlin AF, et al. Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J Exp Med. 2009;206(8):1691–9. doi: 10.1084/jem.20090691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carlin AF, et al. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J Bacteriol. 2007;189(4):1231–7. doi: 10.1128/JB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carlin AF, et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113(14):3333–6. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Costa A, et al. Activation of the NLRP3 inflammasome by group B streptococci. J Immunol. 2012;188(4):1953–60. doi: 10.4049/jimmunol.1102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mohammadi N, et al. Neutrophils Directly Recognize Group B Streptococci and Contribute to Interleukin-1beta Production during Infection. PLoS One. 2016;11(8):e0160249. doi: 10.1371/journal.pone.0160249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McAdams RM, et al. Choriodecidual infection downregulates angiogenesis and morphogenesis pathways in fetal lungs from Macaca nemestrina. PLoS One. 2012;7(10):e46863. doi: 10.1371/journal.pone.0046863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McAdams RM, et al. Choriodecidual Group B Streptococcal Infection Induces miR-155-5p in the Fetal Lung in Macaca nemestrina. Infect Immun. 2015;83(10):3909–17. doi: 10.1128/IAI.00695-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hansen SM, et al. Dynamics of Streptococcus agalactiae colonization in women during and after pregnancy and in their infants. J Clin Microbiol. 2004;42(1):83–9. doi: 10.1128/JCM.42.1.83-89.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schrag SJ, et al. Epidemiology of Invasive Early-Onset Neonatal Sepsis, 2005 to 2014. Pediatrics. 2016;138(6):e20162013. doi: 10.1542/peds.2016-2013. [DOI] [PubMed] [Google Scholar]
- 109.Romero R, et al. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 110.Romero R, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol. 1992;166(1 Pt 1):129–33. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 111.Adams Waldorf KM, Rubens CE, Gravett MG. Use of nonhuman primate models to investigate mechanisms of infection-associated preterm birth. BJOG. 2011;118(2):136–44. doi: 10.1111/j.1471-0528.2010.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Racicot K, et al. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol. 2013;191(2):934–41. doi: 10.4049/jimmunol.1300661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8(1):39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bokulich NA, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ortiz L, et al. Effect of two probiotic strains of Lactobacillus on in vitro adherence of Listeria monocytogenes, Streptococcus agalactiae, and Staphylococcus aureus to vaginal epithelial cells. Curr Microbiol. 2014;68(6):679–84. doi: 10.1007/s00284-014-0524-9. [DOI] [PubMed] [Google Scholar]
- 116.De Gregorio PR, et al. Preventive effect of Lactobacillus reuteri CRL1324 on Group B Streptococcus vaginal colonization in an experimental mouse model. J Appl Microbiol. 2015;118(4):1034–47. doi: 10.1111/jam.12739. [DOI] [PubMed] [Google Scholar]
- 117.De Gregorio PR, Juarez Tomas MS, Nader-Macias ME. Immunomodulation of Lactobacillus reuteri CRL1324 on Group B Streptococcus Vaginal Colonization in a Murine Experimental Model. Am J Reprod Immunol. 2016;75(1):23–35. doi: 10.1111/aji.12445. [DOI] [PubMed] [Google Scholar]
- 118.Patras KA, et al. Streptococcus salivarius K12 Limits Group B Streptococcus Vaginal Colonization. Infect Immun. 2015;83(9):3438–44. doi: 10.1128/IAI.00409-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bernardini R, et al. Neonatal protection and preterm birth reduction following maternal group B Streptococcus vaccination in a mouse model. J Matern Fetal Neonatal Med. 2016:1–7. doi: 10.1080/14767058.2016.1265932. [DOI] [PubMed] [Google Scholar]
- 120.Baker JA, et al. Mucosal vaccination promotes clearance of Streptococcus agalactiae vaginal colonization. Vaccine. 2017;35(9):1273–1280. doi: 10.1016/j.vaccine.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fabbrini M, et al. The Protective Value of Maternal Group B Streptococcus Antibodies: Quantitative and Functional Analysis of Naturally Acquired Responses to Capsular Polysaccharides and Pilus Proteins in European Maternal Sera. Clin Infect Dis. 2016;63(6):746–53. doi: 10.1093/cid/ciw377. [DOI] [PubMed] [Google Scholar]
- 122.Dangor Z, et al. Correlates of protection of serotype-specific capsular antibody and invasive Group B Streptococcus disease in South African infants. Vaccine. 2015;33(48):6793–9. doi: 10.1016/j.vaccine.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 123.Dangor Z, et al. Association between maternal Group B Streptococcus surface-protein antibody concentrations and invasive disease in their infants. Expert Rev Vaccines. 2015;14(12):1651–60. doi: 10.1586/14760584.2015.1085307. [DOI] [PubMed] [Google Scholar]
- 124.Kwatra G, et al. Natural acquired humoral immunity against serotype-specific group B Streptococcus rectovaginal colonization acquisition in pregnant women. Clin Microbiol Infect. 2015;21(6):568 e13–21. doi: 10.1016/j.cmi.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 125.Heath PT. Status of vaccine research and development of vaccines for GBS. Vaccine. 2016;34(26):2876–9. doi: 10.1016/j.vaccine.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 126.Kobayashi M, et al. WHO consultation on group B Streptococcus vaccine development: Report from a meeting held on 27–28 April 2016. Vaccine. 2016 doi: 10.1016/j.vaccine.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kobayashi M, et al. Group B Streptococcus vaccine development: present status and future considerations, with emphasis on perspectives for low and middle income countries. F1000Res. 2016;5:2355. doi: 10.12688/f1000research.9363.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ramaswamy SV, et al. Molecular characterization of nontypeable group B Streptococcus. J Clin Microbiol. 2006;44(7):2398–403. doi: 10.1128/JCM.02236-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bellais S, et al. Capsular switching in group B Streptococcus CC17 hypervirulent clone: a future challenge for polysaccharide vaccine development. J Infect Dis. 2012;206(11):1745–52. doi: 10.1093/infdis/jis605. [DOI] [PubMed] [Google Scholar]
- 130.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378(9807):1962–73. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hanage WP. Serotype replacement in invasive pneumococcal disease: where do we go from here? J Infect Dis. 2007;196(9):1282–4. doi: 10.1086/521630. [DOI] [PubMed] [Google Scholar]
- 132.Nishihara Y, et al. Challenges in reducing group B Streptococcus disease in African settings. Arch Dis Child. 2017;102(1):72–77. doi: 10.1136/archdischild-2016-311419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim SY, et al. Cost-effectiveness of a potential group B streptococcal vaccine program for pregnant women in South Africa. Vaccine. 2014;32(17):1954–63. doi: 10.1016/j.vaccine.2014.01.062. [DOI] [PubMed] [Google Scholar]
- 134.Six A, et al. Srr2, a multifaceted adhesin expressed by ST-17 hypervirulent Group B Streptococcus involved in binding to both fibrinogen and plasminogen. Mol Microbiol. 2015;97(6):1209–22. doi: 10.1111/mmi.13097. [DOI] [PubMed] [Google Scholar]
- 135.Faralla C, et al. Analysis of two-component systems in group B Streptococcus shows that RgfAC and the novel FspSR modulate virulence and bacterial fitness. MBio. 2014;5(3):e00870–14. doi: 10.1128/mBio.00870-14. [DOI] [PMC free article] [PubMed] [Google Scholar]