Figure 8. Bifurcating electron-transfer pathways and transient-absorption dynamics of CPD repair by three types of photolyases.
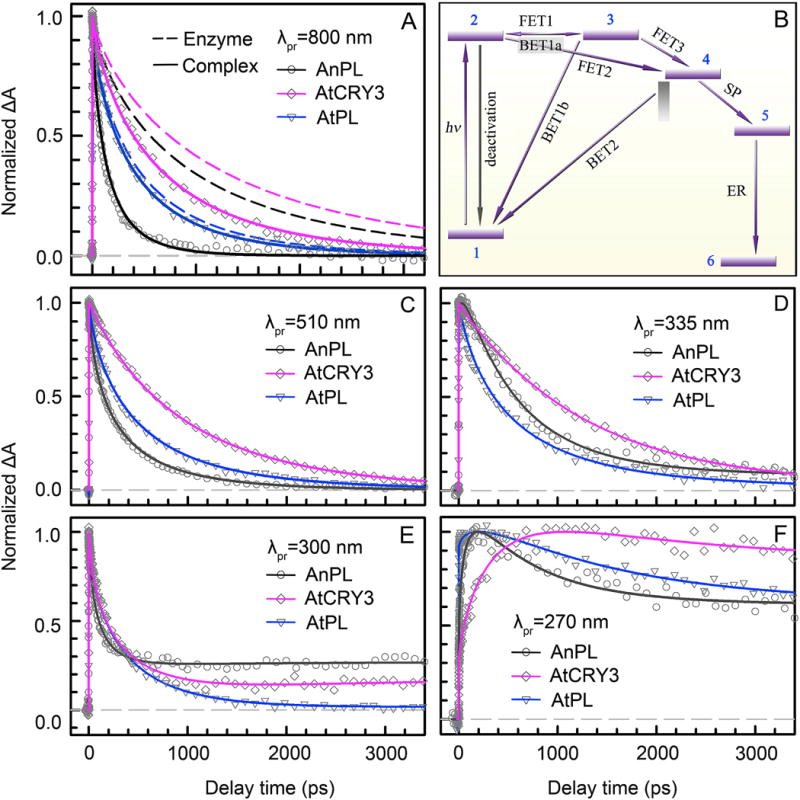
(A) Absorption transients of AnPL, AtCRY3 and AtPL enzymes (dashed lines) and their enzyme-CPD complexes (solid lines) probed at 800 nm for the pure excited-state (LfH−*) decay. In the presence of substrate, the LfH−* dynamics of AnPL becomes drastically faster due to fast direct electron tunneling to the substrate; the change of the LfH−* dynamics is much less in ssDNA PL AtCRY3 and is almost negligible in class II AtPL. (B) The universal repair scheme of CPD by photolyases with ten resolved elementary steps, including seven electron-transfer reactions and dimer splitting processes. The initial electron injection is bifurcated into two routes: the direct tunneling to CPD through the intervening adenine via a superexchange mechanism (FET2), and the two-step hopping pathway (FET1, FET3) bridged by the intermediate adenine. 1, LfH−-Ade+T<>T(CPD); 2, LfH−*-Ade+T<>T; 3, LfH•-Ade−+T<>T; 4, LfH•-Ade+T<>T−; 5, LfH•-Ade+T+T−; 6, LfH−-Ade+T+T. (C) Absorption transients of AnPL, AtCRY3 and AtPL complexes probed at 510 nm for the detection of LfH−* and flavin intermediate LfH•. (D–F) Absorption transients of the three complexes probed in the UV region at 335 nm, 300 nm and 270 nm for the detection of all flavin-related species, the thymine intermediates (T-T− and T−) and final products (T).
