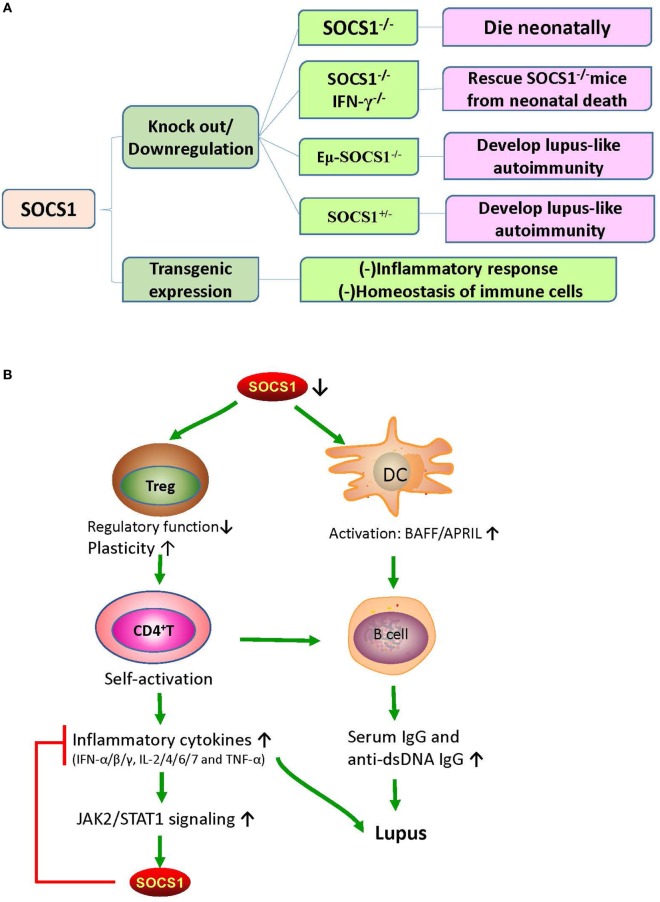
Figure 2.
The effects of SOCS1 abnormalities on murine phenotypes and immune responses. (A) The effects of different SOCS1 level on the immune system of mice model. SOCS1−/− mice died within 3 weeks after birth; and SOCS1−/−IFN-γ−/− prevented the neonatal death of SOCS1−/− mice, thus suggesting that uncontrolled IFN-γ signaling has destructive effects. Eμ-SOCS1−/− and SOCS1+/− mice developed lupus-like autoimmunity with age, indicating that SOCS1 deficiency can initiate an autoimmune response. However, transgenic overexpression of SOCS1 suppresses the immune response and disturbs the homeostasis of immune cells. (B) Roles of SOCS1 in systemic lupus erythematosus (SLE). There is a deficiency in the expression of SOCS1 in SLE. SOCS1-deficient DCs express high levels of BAFF, which leads to abnormal B-cell growth and proliferation. Moreover, low SOCS1 levels correlate with reduced suppressive capacity and enhanced plasticity of Treg cells. These Treg cells maintain high numbers of hyperactivated B cells by promoting the interaction of self-reactive CD4+ T cells with B cells. This interaction leads to the production of diverse inflammatory cytokines and autoantibodies, leading to immune complex formation and tissue injury. Therefore, upregulated SOCS1 levels might play a protective role through the suppression of the destructive response of inflammatory cytokines. APRIL, a proliferation-inducing ligand; BAFF, B-cell activating factor; DC, dendritic cell; IFN, interferon; IL, interleukin; JAK2, Janus kinase 2; SOCS1, suppressor of cytokine signaling 1; STAT1, signal transducer and activator of transcription 1; TNF-α, tumor necrosis factor alpha; Treg, T regulatory cells.