Abstract
Ticks, blood-sucking arthropods, serve as vectors for transmission of infectious diseases including Lyme borreliosis. After tick infestation, several animal species can develop resistance to subsequent infestations, reducing the risk of transmission. In a mouse model, basophils reportedly infiltrate tick-feeding sites during the second but not first infestation and play a crucial role in the expression of acquired tick resistance. However, the mechanism underlying basophil recruitment to the second tick-feeding site remains ill-defined. Here, we investigated cells and their products responsible for the basophil recruitment. Little or no basophil infiltration was detected in T-cell-deficient mice, and adoptive transfer of CD4+ but not CD8+ T cells reconstituted it. Il3 gene expression was highly upregulated at the second tick-feeding site, and adoptive transfer of interleukin-3 (IL-3)-sufficient but not IL-3-deficient CD4+ T cells conferred the basophil infiltration on T-cell-deficient mice, indicating that the CD4+ T-cell-derived IL-3 is essential for the basophil recruitment. Notably, IL-3+ resident CD4+ memory T cells were detected even before the second infestation in previously uninfested skin distant from the first tick-feeding site. Taken together, IL-3 produced locally by skin CD4+ memory T cells appears to play a crucial role in basophil recruitment to the second tick-feeding site.
Keywords: tick infestation, protective immunity, basophils, CD4+ memory T cells, IL-3
Introduction
Ticks are blood-feeding ectoparasites that transmit a variety of pathogenic organisms, such as viruses, bacteria, protozoa, and helminths, many of which can cause various infectious disorders in human and animal hosts (1–3). During blood feeding and salivation, pathogenic microorganisms are delivered from infected ticks to hosts. Tick-borne diseases include viral encephalitis, sever fever with thrombocytopenia syndrome, Lyme disease, monocytic human ehrlichiosis, Rocky mountain spotted fever, and babesiosis (1–3). It has been shown that several animal species, including mice, guinea pigs, rabbits, and bovines, can develop resistance to tick feeding after single or repeated tick infestation, even though the expression of acquired resistance varies, depending the combination of different tick species and animal species/strains (4, 5). The resistance to tick feeding can result in reduced numbers and weights of engorged ticks or death of engorging ticks in re-infestation. From a clinical point of view, this acquired protective immunity to tick infestation is very important, because it reduces the chance of pathogen transmission that causes infectious diseases (6, 7).
Basophils are the least common granulocytes, accounting for less than 1% of peripheral blood leukocytes (8). Tick-feeding sites in guinea pigs with acquired resistance showed substantial infiltration of basophils (9–11). Basophils represent up to 70% of cellular infiltrates in the skin lesion, and basophil depletion with anti-basophil antiserum abolished tick resistance (12), illustrating a crucial role of basophils in acquired tick resistance. Later studies examined the contribution of basophils and mast cells to acquired tick resistance in mice infested with Haemaphysalis longicornis ticks (13–15). H. longicornis principally infests domestic animals such as cattle and is an important vector of pathogens causing babesiosis, Russian encephalitis, and Q fever in animals and humans (16). In mouse model of H. longicornis infestation, no apparent infiltration of basophils was histologically detected in the second tick-feeding site, although mice showed acquired tick resistance (13). Because mast cell-deficient mice failed to show tick resistance (13–15), it was suggested that mast cells, instead of basophils, play a crucial role in the acquired tick resistance in mice, unlike in guinea pigs. We previously revisited the contribution of basophils to acquired protective immunity against tick infestation in mice by using novel analytical tools, including basophil-specific anti-mMCP-8 antibody and engineered mice deficient for only basophils, Mcpt8DTR mice (17). Whereas Giemsa staining of skin sections failed to identify basophil infiltration at H. longicornis-feeding sites, in accordance with earlier reports (13), immunohistochemical analysis with anti-mMCP-8 antibody revealed the basophil accumulation at the second but not first tick-feeding site (17). Importantly, basophil depletion just before the second infestation almost completely abolished the acquired tick resistance (17). Consistent with the previous study, mice deficient for mast cells failed to show acquired tick resistance (17). Thus, both basophils and mast cells are essential for tick resistance in mice. We further demonstrated that IgFc receptors expressed on basophils but not mast cells are essential for the expression of acquired tick resistance (17). Our study, together with the previous study in guinea pig, illustrated that basophils play a pivotal and non-redundant role in antibody-mediated acquired resistance to tick infestation. Nevertheless, it remains totally unknown how basophils are recruited to the second tick-feeding site distant from the first infestation site, leading to tick resistance.
In the present study, we investigated cells and their products responsible for the recruitment of basophils that contribute to acquired tick resistance. We here demonstrate that skin CD4+ memory T cells play an essential role in acquired protective immunity against tick infestations through the production of interleukin-3 (IL-3) that in turn induces basophil recruitment to the tick re-infestation site.
Materials and Methods
Mice
C57BL/6J mice were purchased from Japan SLC. Mcpt8GFP (18), Rag2−/− (19), Il3−/− (20), and Fcer1g−/− (21) mice on the C57BL/6J background were described previously. OT-II Tg/Rag2−/− mice (22) were kindly provided by Dr. Francis R. Carbone. Tcrα−/− mice (23) were obtained from the Jackson laboratory. All mice were maintained under specific pathogen-free conditions in our animal facilities in accordance with the guidelines of the Tokyo Medical and Dental University for animal care, and all animal studies were approved by the Institution Animal Care and Use Committee of Tokyo Medical and Dental University (permit number: 0170087A).
Tick Infestation
Haemaphysalis longicornis of the laboratory-reared strain were used to infest mice. Tick infestation was performed as described previously (17). To examine the tick resistance, mice were infested twice at an interval of 14 days with 40 larvae each time at two different locations, the left flank for the first infestation and the right flank for the second infestation. Most of ticks were detached from the host by day 5 of each infestation.
Antibodies and Reagents
Following reagents were purchased from Biolegend: allophycocyanin (APC)-conjugated anti-CD200R3 (Ba13), anti-CD44 (IM7), Rat IgG2b, and κ (RTK4530); phycoerythrin (PE)-conjugated anti-IL-3 (MP2-8F8), anti-CD63 (NVG-2), Rat IgG1, κ (RTK2071), Rat IgG2a, and κ (RTK2758); fluorescein isothiocyanate (FITC)-conjugated anti-CD49b (HMα2) and anti-CD62L (MEL-14); PE/Cy7-conjugated anti-CD69 (H1.2F3); APC/Cy7-conjugated anti-CD3ε (145-2C11); Pacific Blue™-conjugated anti-CD117 (2B8); brilliant violet 421™-conjugated anti-CD4 (RM4.5); and biotin-conjugated anti-CD4 (GK1.5), anti-CD8a (53-6.7), and recombinant IL-3. Isoflurane and normal rat serum were obtained from WAKO. Anti-CD16/32 mAb (2.4G2) was prepared in our laboratory.
Flow Cytometry
Single-cell suspensions were prepared by treating the flank skin with 125 U/ml collagenase (Wako) at 37°C for 2 h. Isolated cells were preincubated with anti-CD16/32 for 30 min to block non-specific binding of antibodies, subsequently stained with indicated combination of antibodies, and then analyzed with FACSCanto II (BD Biosciences) and FlowJo (Tree star). Each cell lineage was identified as follows: basophils (c-kit−CD49b+CD200R3+), activated basophils (c-kit−CD49b+CD200R3+CD63+), mast cells (c-kit+CD49b+CD200R3+), naive T cells (CD3+CD62L+CD44−), TCM cells (CD3+CD62L+CD44+), TEM cells (CD3+CD62L−CD44+CD69−), and TRM cells (CD3+CD62L+CD44+CD69+). For cytoplasmic staining of cytokines, cells were stimulated for 6 h with phorbol 12-myristate 13-acetate (PMA 0.1 µg/ml; Sigma-Aldrich) plus ionomycin (1 µM; Sigma-Aldrich) in the presence of monensin (BD GolgiStop; BD Biosciences) for the last 2 h. Subsequently, cells were stained with CD3ε, CD4, CD44, CD62L, and CD69 to label surface markers. Cells were then treated with BD Cytofix/Cytoperm Fixation and Permeabilization Solution (BD Biosciences) and stained with anti-IL-3.
RNA Preparation and Real-time Quantitative Reverse Transcription PCR
Total mRNAs from skin tissues were prepared by using RNeasy Mini Kit (Qiagen). cDNAs were synthesized using reverse transcription using ReverTra Ace (TOYOBO) and oligo-dT primers. Quantitative PCR was performed in StepOnePlus™ Real-Time PCR system (Applied Biosystems) using a Fast SYBR Green Master Mix (Applied Biosystems) and the following primer sets:
Gapdh (sense-AGGTCGGTGTGAACGGATTTG and antisense-TGTAGACCATGTAGTTGAGGTCA),
Ifng (sense-ACAGCAAGGCGAAAAAGGATG and antisense-TGGTGGACCACTCGGATGA),
Il3 (sense-GGGATACCCACCGTTTAACCA and antisense-AGGTTTACTCTCCGAAAGCTCTT),
Il4 (sense-ATCATCGGCATTTTGAACGAGG and antisense-TGCAGCTCCATGAGAACACTA),
Il17a (sense-TGTGAAGGTCAACCTCAAAGTC and antisense-AGGGATATCTATCAGGGTCTTCATT).
Il5 (sense-CTCTGTTGACAAGCAATGAGACG and antisense-TCTTCAGTATGTCTAGCCCCTG),
Il13 (sense-GCAACATCACACAAGACCAGA and antisense-GTCAGGGAATCCAGGGCTAC).
The expression of each cytokine gene was normalized by using Gapdh expression as a reference.
Adoptive Transfer of T Cells
Splenic cells isolated from wild-type (WT), Il3−/−, or OT-II Tg/Rag2−/− mice were incubated with biotinylated-anti-CD4 or CD8 antibody at 4°C for 30 min, followed by positive isolation of CD4+ or CD8+ T cells using BD IMag™ (BD Biosciences). T cells (2 × 106 cells per recipient) were intravenously transferred into Rag2−/− mice 24 h before the first infestation.
Continuous Administration of Recombinant IL-3
Rag2−/− mice were treated daily with subcutaneous administration of recombinant IL-3 (100 ng/injection/day) in the right flank and control PBS in the left flank for 10 days from day −7 to day 2, and on day 0, mice were primarily infested with ticks at the administration sites of both flanks.
In Vivo Fluorescent Imaging
Mice were anesthetized with 1.5% isoflurane gas and covered with a heating blanket to keep their body temperature at 37°C. Intravital images of the flank skin were captured with an inverted laser scanning microscope (A1R+; Nikon) equipped with CFI Plan Apochromat λ ×10 or ×20 objective lens or fluorescence stereo microscopes (Leica). NIS elements, Volocity, and Imaris software were used for acquisition and analysis of images.
Statistical Analysis
Statistical analysis was performed with unpaired Student’s t-test. P value <0.05 was considered statistically significant.
Results
Basophils Accumulate at Tick-Feeding Sites at the Early Phase of the Second Tick Infestation in an Antibody-Independent Manner
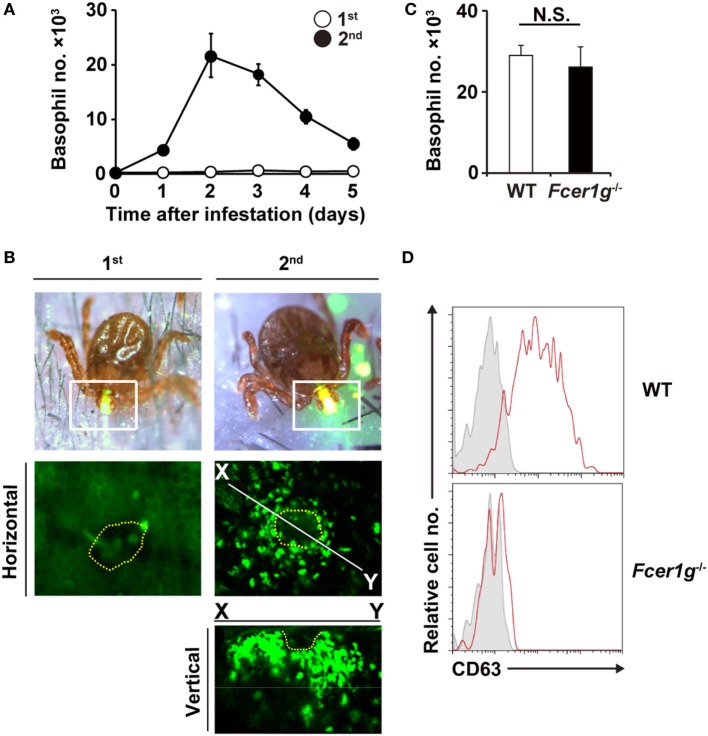
We first examined time course of basophil accumulation at tick-feeding sites during the first and second infestations (Figure 1A). In accordance with our previous study (17), no apparent infiltration of basophils was detected during the first infestation. In contrast, during the second infestation at the skin distant from the first tick infestation site, basophils infiltrated and accumulated in the skin lesion, with a peak of cell number on day 2 or 3 post-infestation (Figure 1A). The decrease of basophil number at the second tick-feeding site after day 3 appeared to coincide with the reduced number of ticks staying attached to the skin due to drop-off of engorged ticks (Figure S1 in Supplementary Material). Intravital imaging, using Mcpt8GFP mice in that only basophils express GFP (18), revealed that green basophils migrated toward and surrounded a tick mouthpart during the second infestation (Figure 1B; Movie S1 in Supplementary Material). In contrast to substantial infiltration of basophils at the second tick-feeding site, the number of mast cells at tick-feeding sites moderately increased during both the first and second infestation (Figure S2 in Supplementary Material).
Figure 1.
Basophils accumulate at tick-feeding sites at the early phase of the second tick infestation in an antibody-independent manner. (A) C57BL/6 mice were infested with larval ticks once or twice at an interval of 14 days. The number of basophils accumulating at tick-feeding sites (mean ± SEM, n = 3 each) was counted at the indicated time points during the first (white circles) and second (black circles) infestations. (B) Mcpt8GFP mice were infested with ticks one or twice and subjected to intravital imaging analysis of green basophils at tick-feeding sites on day 2 of the first or second infestation. Dashed yellow lines indicate the places where tick mouthparts were inserted into the skin. (C,D) Wild-type (WT) or Fcer1g−/− mice were infested twice with ticks. The number of basophils at tick-feeding sites [(C), mean ± SEM, n = 3 each] and their surface expression of CD63 (D) were examined on day 2 of the second infestation. Shaded histograms indicate staining with isotype-matched control antibody. All the data shown are representative of three independent experiments. N.S., not significant.
We previously reported that basophil depletion prior to the second infestation completely abolished the resistance against tick feeding (17), illustrating the pivotal role of basophils in acquired tick resistance (17). Mice deficient for B cells (μMT mice) failed to show the acquired tick resistance in the second infestation (17). This was also the case in mice deficient for FcεRI-γ chain (Fcer1g−/− mice) that lack high-affinity IgE receptor and activating IgG receptors (17). These data demonstrated that antibodies are involved in the manifestation of tick resistance. Of note, basophil accumulation at the second tick-feeding site was normally detected in μMT mice (17), suggesting antibody-independent basophil recruitment. In the present study, we first examined this assumption by analyzing Fcer1g−/− mice. These mice showed basophil accumulation at the second tick-feeding site to an extent comparable to that detected in WT mice (Figure 1C), further supporting the dispensable role of antibody in basophil recruitment. Importantly, increased expression of CD63 was detected on the surface of basophils accumulating at the second tick-feeding site of WT but not Fcer1g−/− mice (Figure 1D). These results suggest that FcεRI-γ-deficient basophils can be recruited to the tick-feeding site but not activated to inhibit tick feeding due to the lack of stimulation with tick antigens and specific antibodies.
CD4+ T Cells Are Essential for Basophil Accumulation at the Second Tick-Feeding Site
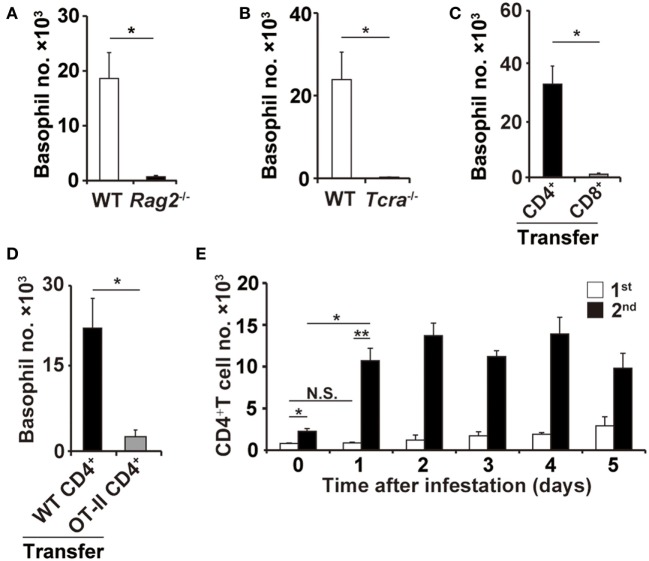
In contrast to μMT and Fcer1g−/− mice, Rag2−/− mice deficient for both T and B cells showed little or no accumulation of basophils at the second tick-feeding site (Figure 2A), suggesting the contribution of T cells to basophil recruitment. Indeed, the basophil accumulation was not detected in Tcra−/− mice deficient for T-cell receptor α chain (Figure 2B). Adoptive transfer of CD4+ T cells but not CD8+ T cells isolated from WT mice conferred the basophil accumulation at the second tick-feeding site on Rag2−/− mice (Figure 2C), indicating the important role of CD4+ T cells in the basophil accumulation. Such basophil accumulation was not detected at the first tick-feeding site unlike at the second tick-feeding site (Figure S3 in Supplementary Material). Of note, adoptive transfer of ovalbumin-specific CD4+ T cells isolated from OT-II transgenic mice failed to reconstitute the basophil accumulation at the second tick-feeding site in Rag2−/− mice (Figure 2D), implying the importance of antigen specificity of CD4+ T cells in promoting basophil recruitment. Adoptive transfer of CD4+ T cells isolated from previously tick-infested WT mice but not from uninfested mice could confer basophil accumulation at the tick-feeding site even in the first infestation on Rag2−/− mice (Figure S4 in Supplementary Material), suggesting CD4+ memory T cells, most likely carrying specificity to tick saliva antigens, may be crucial for triggering basophil recruitment to the tick-feeding site. In accordance with this assumption, the number of CD4+ T cells in the tick-feeding site increased as early as on day 1 of the second but not first tick infestation (Figure 2E).
Figure 2.
CD4+ T cells are essential for basophil accumulation at the second tick-feeding site. (A,B) Wild-type (WT), Rag2−/−, or Tcra−/− mice were infested twice with ticks. The number of basophils (mean ± SEM, n = 3 each) at the second tick-feeding site in each mouse strain was examined on day 2 of infestation. (C) CD4+ or CD8+ T cells were prepared from the spleen of WT mice and adoptively transferred to Rag2−/− mice. Recipient mice were infested twice with ticks, and the basophil number at the second tick-feeding site (mean ± SEM, n = 4 each) was examined as in (A,B). (D) CD4+ T cells isolated from WT or OT-II Tg/Rag2−/− mice were adoptively transferred to Rag2−/− mice, and the basophil number (mean ± SEM, n = 4 each) at the second tick-feeding site of recipient mice was examined as in (C). (E) WT mice were infested once or twice with ticks, and the number of CD4+ T cells at the first (white bars) and second (black bars) tick-feeding sites was examined (mean ± SEM, n = 3 each). All the data shown are representative of three independent experiments. N.S., not significant; *p < 0.05; **p < 0.01.
IL-3 Is Necessary and Sufficient for Basophil Accumulation at the Tick-Feeding Site
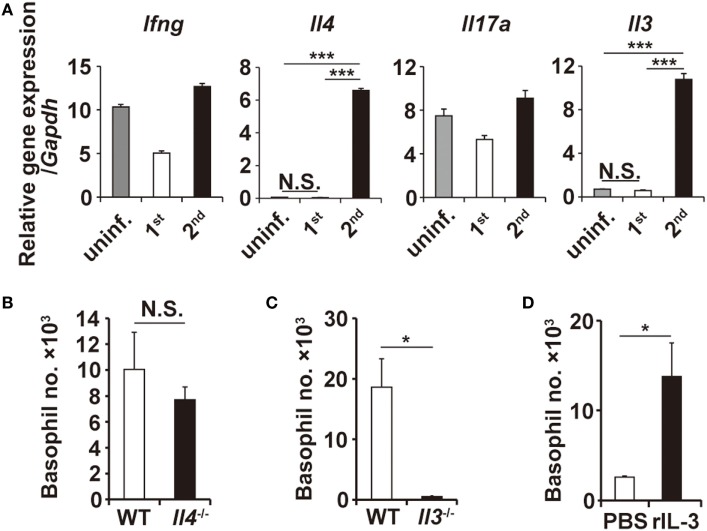
T-cell-derived cytokines reportedly contribute to the recruitment of granulocytes (24–26). RT-PCR analysis revealed that the transcription of Il3 and Il4 genes, among the genes analyzed, was highly upregulated at the tick-feeding site during the second but not first infestation, compared to that in uninfested skin (Figure 3A; Figure S5 in Supplementary Material). To examine the possible contribution of IL-3 and IL-4 to the skin infiltration of basophils, mice deficient for either IL-3 or IL-4 were infested with ticks twice. Basophil accumulation at the second tick-feeding site was detected in IL-4-deficient mice to an extent comparable to that observed in WT mice (Figure 3B). By contrast, little or no accumulation was detected in IL-3-deficient mice (Figure 3C), indicating the essential role of IL-3 for the basophil accumulation. We next explored whether local production of IL-3 in the tick-feeding site is sufficient for basophil recruitment and accumulation. To this end, Rag2−/− mice were treated daily with subcutaneous administration of recombinant IL-3 in the right flank and control PBS in the left flank for 10 days from day −7 to day 2, and on day 0, mice were primarily infested with ticks at the administration sites of both flanks. Even in the absence of T cells, significant accumulation of basophils was detected at the IL-3-treated but not PBS-treated flank during the first infestation (Figure 3D; Figure S6A in Supplementary Material). These results strongly suggested that IL-3 produced locally at the tick-feeding site is necessary and sufficient for basophil recruitment and accumulation, even though we cannot formally exclude the possibility that other factors may modulate them.
Figure 3.
Interleukin-3 (IL-3) is necessary and sufficient for basophil accumulation at the tick-feeding site. (A) The transcriptional expression of indicated genes at uninfested skin, first or second tick-feeding site on day 2 of infestation is shown (mean ± SEM, n = 3 each). (B,C) Wild-type, Il4−/−, or Il3−/− mice were infested twice with ticks, and the number of basophils (mean ± SEM, n = 4 each) at the second tick-feeding site in each mouse strain was examined on day 2 of infestation. (D) Rag2−/− mice were treated daily with subcutaneous administration of recombinant IL-3 (100 ng/injection/day) in the right flank and control PBS in the left flank for 10 days from day −7 to day 2, and on day 0, mice were primarily infested with ticks at the administration sites of both flanks. The number of basophils (mean ± SEM, n = 4 each) at the first tick-feeding site of each side of flank was examined on day 2 of infestation. All the data shown are representative of three independent experiments. N.S., not significant; *p < 0.05; ***p < 0.001.
IL-3 Produced by CD4+ T Cells Is Responsible for Basophil Accumulation at the Second Tick-Feeding Site
The findings that both CD4+ T cells and IL-3 are important for the basophil accumulation prompted us to examine the possibility that IL-3 produced by CD4+ T cells is responsible for the basophil accumulation at the second tick-feeding site. Adoptive transfer of CD4+ T cells from IL-3-deficient mice, unlike those from WT mice, failed to confer the basophil accumulation at the second tick-feeding site on Rag2−/− mice, even though the number of IL-3-deficient CD4+ T cells accumulating at the tick-feeding site was comparable to that of WT CD4+ T cells (Figure 4A; Figure S6B in Supplementary Material). The ex vivo stimulation of CD4+ T cells isolated from the tick-feeding site, followed by intracellular staining for IL-3, revealed that the number of CD4+ T cells with IL-3-producing capacity (hereafter referred to as IL-3+CD4+ T cells) significantly increased at the tick-feeding site during the second infestation compared to that during the first infestation (Figure 4B, left panel), in parallel with the extent of basophil accumulation at the tick-feeding site. The frequency of IL-3-producing cells among CD4+ T cells at the second tick-feeding site on day 2 of infestation was 1–5% (Figure 4B, right panel). All these results suggested that a fraction of CD4+ T cells at the second tick-feeding site can produce IL-3 that in turn induces basophil infiltration into the skin lesion.
Figure 4.
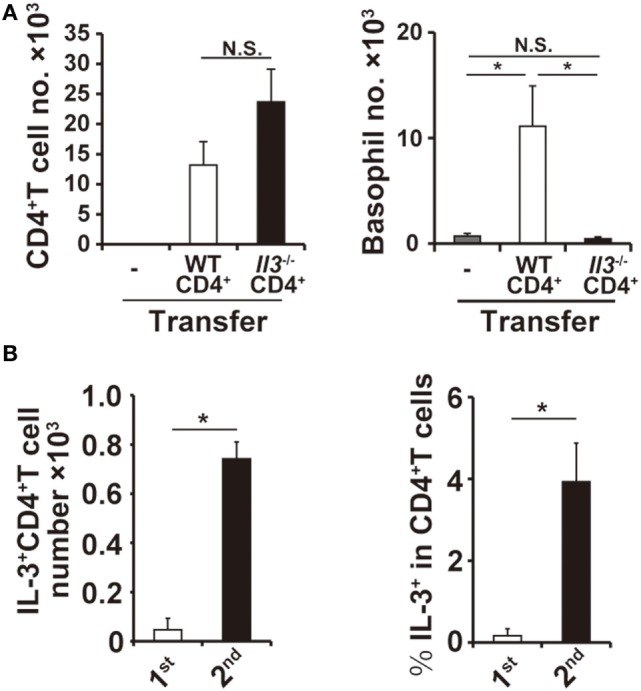
Interleukin-3 (IL-3) produced by CD4+ T cells is responsible for basophil accumulation at the second tick-feeding site. (A) Splenic CD4+ T cells isolated from wild-type (WT) or Il3−/− mice were adoptively transferred into Rag2−/− mice, and recipient mice were infested twice with ticks. The number of CD4+ T cells (left panel) and basophils (right panel) at the second tick-feeding site was examined on day 2 of infestation (mean ± SEM, n = 4 each). (B) WT mice were infested twice with ticks, and the number of IL-3+CD4+ T cells at the first and second tick-feeding sites (left panel) and the frequency of IL-3+ T cells among CD4+ T cells at the first and second tick-feeding sites (right panel) were determined (mean ± SEM, n = 3 each). All the data shown are representative of three independent experiments. N.S., not significant; *p < 0.05.
IL-3+ CD4+ T Cells at the Second Tick-Feeding Site Show a Memory Phenotype, Including Resident and Effector Memory T Cells
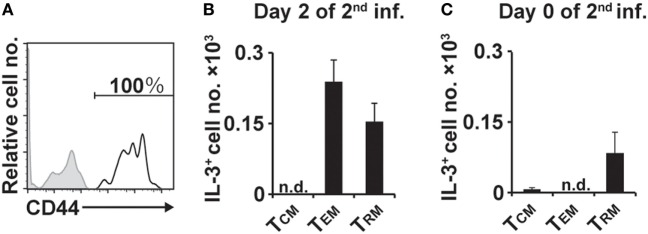
We next examined the surface phenotype of IL-3+CD4+ T cells at the second tick-feeding site. Virtually all of them present on day 2 of the second infestation expressed CD44 (Figure 5A), suggesting that they are memory T cells (27). CD4+ memory T cells include central memory (TCM), effector memory (TEM), and resident memory (TRM) cells (28–32). Among IL-3+CD44+CD4+ T cells present at the second tick-feeding site on day 2 of infestation, approximately 60 and 40% showed the surface phenotype of TEM and TRM, respectively, and T cells with the TCM phenotype was barely detected (Figure 5B). Notably, IL-3+CD4+ T cells with the TRM but not TEM phenotype were detected on day 0 of the second infestation, namely 14 days after the initiation of the first infestation and immediately before the second infestation (Figure 5C). Such IL-3+CD4+ T cells with the TRM phenotype were hardly detected in the skin of naive mice without any experience of tick infestation (data not shown). When CD4+ T cells isolated from uninfested WT mice were adoptively transferred into Rag2−/− mice, some of them were detected as TRM cells at previously uninfested skin on day 14 of the first infestation (Figure S7 in Supplementary Material). These results suggested that after the first infestation, IL-3+CD4+ TRM cells, most likely carrying specificity to tick antigens, were generated and widely distributed to the skin distant from the first tick-feeding site.
Figure 5.
Interleukin-3 (IL-3)+CD4+ T cells at the second tick-feeding site show memory phenotypes, including TRM and TEM cells. (A) The expression of CD44 (open histogram) on IL-3+CD4+ T cells accumulating at the second tick-feeding site of wild-type mice is shown. A shaded histogram indicates staining with isotype-matched control antibody. (B,C) The number of IL-3+CD4+ T cells showing the phenotype of TCM (CD3+CD62L+CD44+), TEM (CD3+CD62L−CD44+CD69−), or TRM (CD44+CD62L−CD69+) on day 2 (B) and on day 0 (C) of the second infestation is shown (mean ± SEM, n = 3 each). All the data shown are representative of three independent experiments. n.d., not detected.
Discussion
Basophils have been shown to infiltrate and accumulate at tick-feeding sites during re-infestation but not the first infestation and are critically involved in the manifestation of acquired resistance to tick feeding (12, 17). However, the mechanism underlying basophil recruitment to the second tick-feeding site, far from the first infestation site, remained ill-defined. In the present study, we identified cells and their product that are responsible for the basophil recruitment. Adoptive transfer experiments revealed that IL-3 produced by CD4+ T cells is essential for the basophil recruitment to the second tick-feeding site. IL-3+CD4+ T cells were readily detected at the second but not first tick-feeding site, and virtually all of them showed the memory phonotype. Importantly, IL-3+CD4+ TRM cells were detected in previously uninfested skin, distant from the first tick-feeding site, of previously infested mice. Thus, skin CD4+ memory T cells play a pivotal role in acquired tick resistance through local production of IL-3, most likely in response to stimulation with tick antigens, that in turn promotes skin infiltration of basophils necessary for the manifestation of resistance to tick feeding.
TRM cells are a recently described subset of memory T cells that stay long term in peripheral tissues, in contrast to circulating memory T-cell subsets including TCM and TEM cells (29–32). TRM cells are derived from precursor cells, which entered tissues during the effector phase of primary response and stayed within the tissues. Therefore, they can respond readily to pathogen challenge at the sites independently of recruitment of blood-circulating T cells. TRM cells have been extensively characterized among the CD8+ T-cell subset that plays a crucial role in protection from viral infections. CD8+ TRM cells have been shown to directly control local infection, including killing of infected cells, and indirectly modify the tissue environment to promote inflammation (29–32). Recent studies reported the presence of CD4+ TRM cells in peripheral tissues such as the lung, genital tract, and skin (33–37), even though less is known about their functions compared to those of CD8+ TRM cells. Skin-resident, IFNγ-producing CD4+ memory T cells protect against Leishmania major re-infection by recruiting circulating T cells and inflammatory monocytes to the site of re-infection, leading to efficient control of parasitic infections (38, 39). In the present study, we demonstrated for the first time that IL-3-producing CD4+ memory T cells, including TRM cells, play an essential role in the basophil recruitment to the site of tick re-infestation and, thereby, contribute to the acquired protective immunity against tick infestation. Even before the second tick infestation, IL-3+CD4+ TRM cells could be detected in previously uninfested skin far from the first tick-feeding site, suggesting that CD4+ effector T cells generated during the first infestation migrated into the skin throughout the body, and some of them were retained as TRM cells in the skin. The number of IL-3+CD4+ TRM cells at the second tick-feeding site increased up to two-fold during the first 2 days of re-infestation (Figures 5B,C). IL-3+CD4+ TEM cells were barely detected in the skin on day 0 of the second infestation but rapidly accumulated by day 2 (Figures 5B,C), indicating that circulating CD4+ TEM cells were recruited to the second tick-feeding site. Although it remains to be investigated whether CD4+ TRM cells contribute to the recruitment of circulating CD4+ TEM cells as reported in the L. major re-infection (38), IL-3+CD4+ TEM cells recruited to the second tick-feeding site, in addition to pre-existing IL-3+CD4+ TRM cells, appear to further promote the basophil recruitment to the skin. We showed here the importance of IL-3 produced by skin CD4+ memory T cells in acquired anti-tick immunity while IFNγ produced by skin CD4+ memory T cells plays an important role in acquired immunity against Leishmania infection (38). This suggests that skin CD4+ memory T cells may utilize distinct cytokines to combat with different species of parasites.
A previous study reported that basophils are transiently recruited to draining lymph nodes when mice are primarily infected with helminth Nippostrongylus brasiliensis (40). Similarly, we detected basophil recruitment to draining lymph nodes during the first tick infestation (17). In contrast, basophil recruitment to N. brasiliensis-infected skin lesions was detected during the second but not first infections (41), as observed in the tick-infested skin. Taking together, the basophil recruitment to draining lymph nodes occurs in the primary immune response to the parasitic infections whereas that to the skin takes place only in recall response. It has been shown that IL-3 is required for basophil recruitment to draining lymph nodes in primary N. brasiliensis infection and CD4+ T cells are the major producer of IL-3 (40). Therefore, the requirement of IL-3 produced by CD4+ T cells appears to be common to the basophil recruitment to draining lymph nodes and skin in the parasitic infections, in spite of the difference in the timing of recruitment. Of note, IL-3+CD4+ T cells were detected in draining lymph nodes during the first tick infestation (data not shown), whereas they were detectable at the skin during the second but not first infestation, suggesting the importance of CD4+ memory T cells in the skin but not in lymph nodes. Indeed, IL-3+CD4+ T cells present in draining lymph nodes during the first tick infestation expressed low levels of CD44 (data not shown) in contrast to IL-3+CD4+ T cells in the second tick-feeding site. Such IL-3+CD4+-naive T cells were barely detected in the skin, unlikely in draining lymph nodes, during the first tick infestation (data not shown). Taken together, the phenotypic difference of IL-3+CD4+ T cells, naive versus memory, as well as their distinct localization appears to account for the different timing of basophil recruitment in the skin and lymph nodes. Of note, another study showed that inhalation of Aspergillus fumigatus extract induced prompt increase in basophil numbers in the lung in a manner independent of T cells and IL-3 (42), suggesting that T-cell-derived IL-3 is not always the absolute requirement for basophil recruitment to peripheral tissues.
The exact mechanism by which IL-3 promotes basophil recruitment to the skin in tick re-infestation remains to be investigated. Previous studies demonstrated that endothelial cells express IL-3 receptor, and IL-3 can activate them to promote rolling and adhesion of basophils on endothelium in an adhesion molecule- and chemokine-dependent manner (43, 44). IL-3 also acts on basophils to enhance their adhesion onto endothelium (45). Therefore, IL-3 appears to induce tissue infiltration of basophils at least in part by promoting basophil adhesion to endothelium, leading to transendothelial migration. Our data suggested that IL-3 may also contribute to the accumulation of neutrophils at the tick-feeding site (Figure S6 in Supplementary Material).
In conclusion, we have defined the mechanism by which basophils infiltrate the skin of tick re-infestation. Skin CD4+ memory T cells play an essential role in acquired protective immunity to tick infestation through the production of IL-3 that in turn induces basophil recruitment to the site of tick re-infestation. This illustrates the excellent collaboration of T cells and basophils, belonging to the adaptive and innate immune systems, respectively, for protection against ticks that cause serious infectious diseases.
Ethics Statement
This study was carried out in accordance with the guidelines of the Tokyo Medical and Dental University for animal care. The protocol was approved by the Institution Animal Care and Use Committee of Tokyo Medical and Dental University.
Author Contributions
TO, SY, MO, YK, KM, YY, HiK, NW, and HaK designed the research. TO, SY, and YT performed experiments and analyzed data. KY, KI, and YT prepared ticks suitable for infestation. SY, HS, CT, and HY generated Mcpt8GFP mice. HaK and SY supervised the work. TO, SY, and HaK wrote the manuscript. All authors provided critical review of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Francis R. Carbone from the University of Melbourne for providing OT-II Tg/Rag2−/− mice, R. Sasaki, and H. Ohtsuka for technical assistance.
Footnotes
Funding. This work was supported by research grants from Japanese Ministry of Education, Culture, Sports, Science and Technology [15H05786 (HaK) and 17K15719 (SY)], Ohyama Health Foundation [X2658 (SY)], and the Joint Usage/Research Program of Medical Research Institute, Tokyo Medical and Dental University (SY).
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/article/10.3389/fimmu.2017.01348/full#supplementary-material.
Basophils accumulate around a tick mouthpart at the second tick-feeding site. Mcpt8GFP mice were infested twice with ticks and subjected to intravital imaging analysis of green basophils at tick-feeding sites on day 2 of the second infestation as in Figure 1B. Images were taken at 2 min intervals for 1.5 h.
Abbreviations
IL-3, interleukin-3; TRM, tissue-resident memory T; TEM, effector memory T; TCM, central memory T.
References
- 1.Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis (2001) 32(6):897–928. 10.1086/319347 [DOI] [PubMed] [Google Scholar]
- 2.de la Fuente J, Estrada-Pena A, Venzal JM, Kocan KM, Sonenshine DE. Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci (2008) 13:6938–46. 10.2741/3200 [DOI] [PubMed] [Google Scholar]
- 3.Saito T, Fukushima K, Umeki K, Nakajima K. Severe fever with thrombocytopenia syndrome in Japan and public health communication. Emerg Infect Dis (2015) 21(3):487–9. 10.3201/eid2103.140831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wikel SK. Host immunity to ticks. Annu Rev Entomol (1996) 41(1):1–22. 10.1146/annurev.en.41.010196.000245 [DOI] [PubMed] [Google Scholar]
- 5.Dizij A, Kurtenbach K. Clethrionomys glareolus, but not Apodemus flavicollis, acquires resistance to Ixodes ricinus L., the main European vector of Borrelia burgdorferi. Parasite Immunol (1995) 17(4):177–83. 10.1111/j.1365-3024.1995.tb00887.x [DOI] [PubMed] [Google Scholar]
- 6.Wikel SK. Host resistance to tick-borne pathogens by virtue of resistance to tick infestation. Ann Trop Med Parasitol (1980) 74(1):103–4. 10.1080/00034983.1980.11687318 [DOI] [PubMed] [Google Scholar]
- 7.Jones LD, Nuttall PA. The effect of host resistance to tick infestation on the transmission of Thogoto virus by ticks. J Gen Virol (1990) 71(5):1039–43. 10.1099/0022-1317-71-5-1039 [DOI] [PubMed] [Google Scholar]
- 8.Galli SJ. Mast cells and basophils. Curr Opin Hematol (2000) 7(1):32–9. 10.1097/00062752-200001000-00007 [DOI] [PubMed] [Google Scholar]
- 9.Allen JR. Tick resistance: basophils in skin reactions of resistant guinea pigs. Int J Parasitol (1973) 3(2):195–200. 10.1016/0020-7519(73)90024-6 [DOI] [PubMed] [Google Scholar]
- 10.Brown SJ, Askenase PW. Cutaneous basophil responses and immune resistance of guinea pigs to ticks: passive transfer with peritoneal exudate cells or serum. J Immunol (1981) 127(5):2163–7. [PubMed] [Google Scholar]
- 11.Askenase PW. Immunopathology of parasitic diseases: involvement of basophils and mast cells. Springer Semin Immunopathol (1980) 2(4):417–42. 10.1007/BF01857177 [DOI] [Google Scholar]
- 12.Brown SJ, Galli SJ, Gleich GJ, Askenase PW. Ablation of immunity to Amblyomma americanum by anti-basophil serum: cooperation between basophils and eosinophils in expression of immunity to ectoparasites (ticks) in guinea pigs. J Immunol (1982) 129(2):790–6. [PubMed] [Google Scholar]
- 13.Matsuda H, Fukui K, Kiso Y, Kitamura Y. Inability of genetically mast cell-deficient W/Wv mice to acquire resistance against larval Haemaphysalis longicornis ticks. J Parasitol (1985) 71(4):443–8. 10.2307/3281535 [DOI] [PubMed] [Google Scholar]
- 14.Matsuda H, Nakano T, Kiso Y, Kitamura Y. Normalization of anti-tick response of mast cell-deficient W/Wv mice by intracutaneous injection of cultured mast cells. J Parasitol (1987) 73(1):155–60. 10.2307/3282361 [DOI] [PubMed] [Google Scholar]
- 15.Matsuda H, Watanabe N, Kiso Y, Hirota S, Ushio H, Kannan Y, et al. Necessity of IgE antibodies and mast cells for manifestation of resistance against larval Haemaphysalis longicornis ticks in mice. J Immunol (1990) 144(1):259–62. [PubMed] [Google Scholar]
- 16.Tsuji N, Miyoshi T, Battsetseg B, Matsuo T, Xuan X, Fujisaki K. A cysteine protease is critical for Babesia spp. transmission in Haemaphysalis ticks. PLoS Pathog (2008) 4(5):e1000062. 10.1371/journal.ppat.1000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest (2010) 120(8):2867–75. 10.1172/JCI42680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyake K, Shiozawa N, Nagao T, Yoshikawa S, Yamanishi Y, Karasuyama H. Trogocytosis of peptide-MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc Natl Acad Sci U S A (2017) 114(5):1111–6. 10.1073/pnas.1615973114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell (1992) 68(5):855–67. 10.1016/0092-8674(92)90029-C [DOI] [PubMed] [Google Scholar]
- 20.Nishinakamura R, Miyajima A, Mee P, Tybulewicz V, Murray R. Hematopoiesis in mice lacking the entire granulocyte-macrophage colony-stimulating factor/interleukin-3/interleukin-5 functions. Blood (1996) 88(7):2458–64. [PubMed] [Google Scholar]
- 21.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR γ chain deletion results in pleiotrophic effector cell defects. Cell (1994) 76(3):519–29. 10.1016/0092-8674(94)90115-5 [DOI] [PubMed] [Google Scholar]
- 22.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol (1998) 76(1):34–40. 10.1046/j.1440-1711.1998.00709.x [DOI] [PubMed] [Google Scholar]
- 23.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature (1992) 360(6401):225–31. 10.1038/360225a0 [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol (2007) 119(6):1303–10. 10.1016/j.jaci.2007.03.048 [DOI] [PubMed] [Google Scholar]
- 25.Chen K, Kolls JK. Interluekin-17A (IL17A). Gene (2017) 614:8–14. 10.1016/j.gene.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehm U, Klamp T, Groot M, Howard JC. Celluar responses to interferon-gamma. Annu Rev Immunol (1997) 15(1):749–95. 10.1146/annurev.immunol.15.1.749 [DOI] [PubMed] [Google Scholar]
- 27.Baaten BJG, Li C-R, Bradley LM. Multifaceted regulation of T cells by CD44. Commun Integr Biol (2010) 3(6):508–12. 10.4161/cib.3.6.13495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature (1999) 401(6754):708–12. 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- 29.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol (2013) 31(1):137–61. 10.1146/annurev-immunol-032712-095954 [DOI] [PubMed] [Google Scholar]
- 30.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med (2015) 21(7):688–97. 10.1038/nm.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol (2016) 16(2):79–89. 10.1038/nri.2015.3 [DOI] [PubMed] [Google Scholar]
- 32.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity (2014) 41(6):886–97. 10.1016/j.immuni.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science (2014) 346(6205):93–8. 10.1126/science.1257530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol (2011) 187(11):5510–4. 10.4049/jimmunol.1102243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity (2014) 41(4):633–45. 10.1016/j.immuni.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins N, Jiang X, Zaid A, Macleod BL, Li J, Park CO, et al. Skin CD4+ memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat Commun (2016) 7:11514. 10.1038/ncomms11514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4+ T cells that persist in the lungs. J Exp Med (2001) 193(8):981–6. 10.1084/jem.193.8.981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med (2015) 212(9):1405–14. 10.1084/jem.20142101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glennie ND, Volk SW, Scott P. Skin-resident CD4+ T cells protect against Leishmania major by recruiting and activating inflammatory monocytes. PLoS Pathog (2017) 13(4):e1006349. 10.1371/journal.ppat.1006349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3 but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol (2010) 184(3):1143–7. 10.4049/jimmunol.0902447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obata-Ninomiya K, Ishiwata K, Tsutsui H, Nei Y, Yoshikawa S, Kawano Y, et al. The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med (2013) 210(12):2583–95. 10.1084/jem.20130761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poddighe D, Mathias CB, Freyschmidt EJ, Kombe D, Caplan B, Marseglia GL, et al. Basophils are rapidly mobilized following initial aeroallergen encounter in naive mice and provide a priming source of IL-4 in adaptive immune responses. J Biol Regul Homeost Agents (2014) 28(1):91–103. [PubMed] [Google Scholar]
- 43.Korpelainen EI, Gamble JR, Vadas MA, Lopez AF. IL-3 receptor expression, regulation and function in cells of the vasculature. Immunol Cell Biol (1996) 74(1):1–7. 10.1038/icb.1996.1 [DOI] [PubMed] [Google Scholar]
- 44.Lim LHK, Burdick MM, Hudson SA, Mustafa FB, Konstantopoulos K, Bochner BS. Stimulation of human endothelium with IL-3 induces selective basophil accumulation in vitro. J Immunol (2006) 176(9):5346–53. 10.4049/jimmunol.176.9.5346 [DOI] [PubMed] [Google Scholar]
- 45.Bochner BS, McKelvey AA, Sterbinsky SA, Hildreth JE, Derse CP, Klunk DA, et al. IL-3 augments adhesiveness for endothelium and CD11b expression in human basophils but not neutrophils. J Immunol (1990) 145(6):1832–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Basophils accumulate around a tick mouthpart at the second tick-feeding site. Mcpt8GFP mice were infested twice with ticks and subjected to intravital imaging analysis of green basophils at tick-feeding sites on day 2 of the second infestation as in Figure 1B. Images were taken at 2 min intervals for 1.5 h.