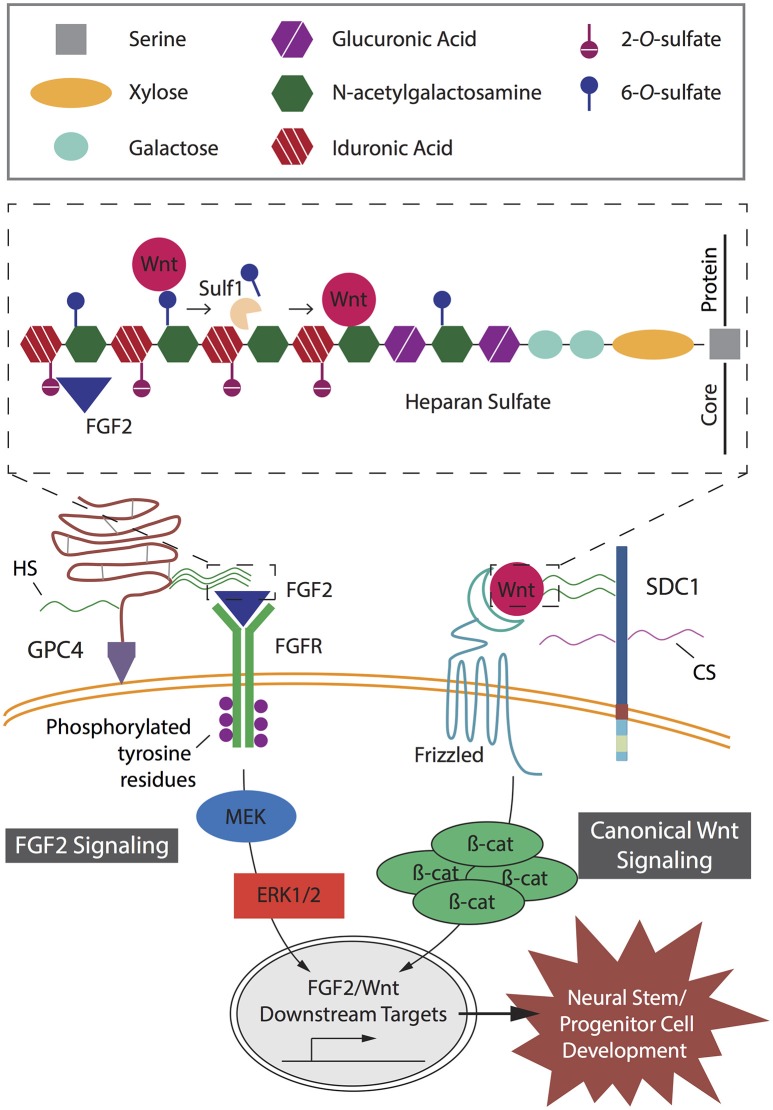
Figure 2.
Key heparan sulfate proteoglycan (HSPG)-mediated signaling pathways in neural stem and progenitor cell (NSPC) development. Fibroblast growth factor (FGF2) and canonical Wnt signaling pathways act independently to mediate proliferation and lineage differentiation of cells with specific interactions determined by specific heparan sulfate chain sulfation profiles. In FGF2 signaling, GPI-anchored HSPG glypican-4 (GPC4) HS chains modulate the binding of FGF2 to its receptor, FGFR. Binding of FGF2 to HS requires 2-O-sulfates (inset). Subsequent phosphorylation of tyrosine residues mediates interactions with cytosolic adaptor proteins; resulting in activation of the MEK/ERK cascade and downstream targets, key transcriptional factors promoting NSPC development. In canonical Wnt signaling, the Wnt ligand binds to its receptor, Frizzled, and is mediated by SDC1 via 6-O-sulfation sites on the HS chains (inset). The presence of HS chain 6-O-sulfation sites enables high affinity binding of Wnt ligands and prevents interaction between Wnts and their Frizzled receptors. In the presence of Sulf1, selective 6-O-sulfates are removed from the HS chain, resulting in low affinity binding of Wnt ligands to the 6-O-desulfated site allowing presentation to the Frizzled receptors. Wnt binding to its receptor leads to accumulation of β-catenin in the cytoplasm, which translocates into the nucleus to activate downstream targets and promote NSPC development.