Abstract
Intake of long-chain omega-3 polyunsaturated fatty acids (LC-n3-PUFA) is commonly recognized to reduce cardiovascular disease (CVD). In previous studies, cold-pressed whale oil (CWO) and cod liver oil (CLO) were given as a dietary supplement to healthy volunteers. Even though CWO contains less than half the amount of LC-n3-PUFA of CLO, CWO supplement resulted in beneficial effects on anti-inflammatory and CVD risk markers compared to CLO. In the present study, we prepared virtually lipid-free extracts from CWO and CLO and evaluated the antioxidative capacity (AOC) and anti-inflammatory effects. Oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays were used to test the AOC, and the results indicated high levels of antioxidants present in all extracts. The anti-inflammatory effects of the extracts were tested with lipopolysaccharide- (LPS-) treated THP-1 cells, measuring its ability to reduce cytokine and chemokine secretion. Several CWO extracts displayed anti-inflammatory activity, and a butyl alcohol extract of CWO most effectively reduced TNF-α (50%, p < 0.05) and MCP-1 (85%, p < 0.001) secretion. This extract maintained a stable effect of reducing MCP-1 secretion (60%, p < 0.05) even after long-term storage. In conclusion, CWO has antioxidant and anti-inflammatory activities that may act in addition to its well-known LC-n3-PUFA effects.
1. Introduction
Atherosclerosis is a cardiovascular disease (CVD) characterized by lipid accumulation and chronic inflammation in the arteries. The atheromatous plaques accumulate over years within the intima of arteries and may ultimately rupture, resulting in atherothrombosis and myocardial infarction [1]. Even though the mortality rate from CVD has decreased in high-income countries during the last decades, CVD remains the leading cause of mortality worldwide [2]. Thus, novel therapies to reduce the atherosclerotic risk and to prevent severe adverse effects associated with prevailing treatments are still needed. In recent years, the inflammatory aspects of atherosclerosis have been thoroughly elucidated [3–5]. Several cytokines and chemokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1), are important contributors in atherogenesis and atherosclerosis [6–10]. Consequently, novel anti-inflammatory components may contribute significantly in both prevention and therapeutic treatment of atherosclerosis. Intake of long-chain omega-3 polyunsaturated fatty acids (LC-n3-PUFA) is a recognized risk reducer of CVD due to the triacylglycerol-lowering and anti-inflammatory effects [11–13]. As LC-n3-PUFA is very susceptible to oxidation, it seems important to eat sufficient amounts of antioxidants to prevent lipid peroxidation [14]. Thus, combining LC-n3-PUFA with other anti-inflammatory agents may be an effective approach to reduce atherosclerosis. Bioprospecting for antioxidants and anti-inflammatory components has led to an extended search in a wide range of marine species, mainly focusing on small organisms such as bacteria, fungi, and invertebrates [15]. In this context, larger marine mammals have received less attention. The minke whale (Balaenoptera acutorostrata) is an Atlantic finback whale regularly migrating to areas in the north where they feed on pelagic fish and crustaceans [16]. Minke whales have a thick layer of blubber [17] which is a vascularized hypodermal adipose tissue, vital for buoyancy, thermal insulation, and energy storage [18]. The blubber is a modified adipose tissue composed of adipocytes and connective tissue comprised of highly organized elastin and collagen fibers [19]. Intact blubber and oil extracted from blubber have been used in the diet in Arctic and Subarctic regions for centuries.
Previous dietary studies of cold-pressed oil from minke whale blubber (CWO) have indicated beneficial effects on CVD markers and improved an anti-inflammatory effect, also in comparison to cod liver oil (CLO) supplementation [20, 21]. Notably, the LC-n3-PUFA content in CWO was less than half when compared to CLO (10.3% versus 25.1%), indicating that the anti-inflammatory effects may rely on putative unknown components from blubber interacting with LC-n3-PUFA [21]. The objective in the present study was hence to elucidate possible in vitro antioxidative and anti-inflammatory effects of lipid-free extracts from CWO using biochemical assays and lipopolysaccharide- (LPS-) stimulated THP-1 cells.
2. Materials and Methods
2.1. Samples
Frozen fresh blubber from the ventral groove of the minke whale was provided from Ellingsen Seafood AS (Skrova, Norway).
2.2. Extraction
The blubber from the minke whale was grinded once before centrifugation at <2000 ×g (<40°C), and the oily top layer was collected (CWO-1). The remnant blubber was centrifuged again at the same speed, and again the oily top layer was collected (CWO-2). For comparison, commercially available CLO [22], rich in LC-n3-PUFA, was included. Each sample was further treated equally; 250 grams was extracted in 800 ml methanol/dichloromethane (1 : 1). The dichloromethane fraction containing most of the lipids was discarded, and residual dichloromethane in the methanol phase was removed by the use of a rotavapor. The remaining oil was removed by 3 × 200 ml heptane liquid-liquid extraction, and the methanol fraction was evaporated to almost dryness prior to addition of 100 ml water (dH2O). Further evaporation removed residual methanol from the extract. After evaporation to almost dryness, the extract was once again redissolved in 100 ml dH2O. The sample, now dissolved in water, was partitioned first with 3 × 200 ml ethyl acetate (EtOAc), then with 3 × 200 ml butyl alcohol (BuOH). The three extracts (EtOAc, BuOH, and H2O) were subsequently evaporated using a rotavapor and, finally, to dryness under a steam of nitrogen. Extracts were stored at −20°C prior to further analyses, and stock solutions (10 mg/ml) were prepared in dH2O and 5% dimethyl sulfoxide (DMSO).
2.3. Oxygen Radical Absorbance Capacity (ORAC)
The antioxidative effect was measured with the ORAC assay [23]. Samples were mixed with the synthetic free radical generator 2,2′-azobis-2-methyl-propanimidamide dihydrochloride (AAPH, Sigma-Aldrich) and the oxidizable fluorescein sodium salt (number F6377, Sigma-Aldrich) at physiological pH. The water-soluble vitamin E equivalent Trolox was used as standard, and fluorescence decay, as a result of the radical attack, was measured at 485 and 520 nm (Spectramax Gemini EM fluorimeter, Molecular Devices, Sunnyvale, USA). The antioxidative capacity (AOC) was defined as the net difference between the areas under the fluorescence decay curves for the sample and blank, respectively. The results are presented as μmol Trolox equivalents (TE)/100 g oil. Extracts were measured at a concentration of 0.1 mg/ml sample.
2.4. Ferric Reducing Antioxidant Power (FRAP)
The antioxidant activities of the extracts were also evaluated using FRAP assay [24]. Samples were incubated together with the FRAP reagents (ferric-tripyridyltriazine complex, pH 3.6) in a microtiter plate for 30 minutes. The intense blue color formed as a result of reduction to the ferrous-tripyridyltriazine complex was measured at 593 nm (Spectramax Gemini EM fluorimeter, Molecular Devices, Sunnyvale, USA). The AOC was determined as TE from the Trolox standard curve (0–1000 μM). The results are presented as μmol TE/100 g oil.
2.5. Differentiated THP-1 Cells
THP-1 (number TIB-202, ATCC) is a monocyte cell line derived from a patient with acute monocytic leukemia. The cell line grows in suspension; however, after treatment with phorbol 12-myristate 13-acetate (PMA), the cells differentiate into adherent macrophage-like cells [25]. The cells were maintained in RPMI-1640 (FG1385, Biochrom) media with 10% fetal bovine serum (FBS) (S0115, Biochrome) and 10 μg/ml gentamicin (A2712, Biochrome). Cells were incubated in 5% CO2 atmosphere and 37°C for all THP-1-based experiments and subcultured every 3-4 days when cell concentration reached 8 × 105 viable cells. Every batch of THP-1 cells was evaluated with dose response against of LPS prior to use.
2.6. Anti-Inflammatory Screening Assay
Approximately 1 × 105 THP-1 cells were seeded out and differentiated with 50 ng/ml PMA (P1585, Sigma-Aldrich) in 96-well plates. After 48 hours of incubation, cells were washed with PBS and fresh RPMI (without PMA) was added. The plates were incubated for 24 hours before the addition of 90 μl fresh RPMI and 10 μl extracts in different concentrations (50 μg/ml, 10 μg/ml, and 1 μg/ml) to the respective wells. After a 1-hour incubation, LPS (L2630, Sigma-Aldrich) was added at a final concentration of 5 ng/ml to all wells except for the negative controls. The plates were then incubated for 6 hours and immediately frozen at −80°C. Negative, positive, and DMSO controls (0.05%) were included in every run.
2.7. ELISA
2.7.1. Tumor Necrosis Factor-Alpha (TNF-α)
One day prior to the ELISA testing of TNF-α secretion, MaxiSorp 96F-well plates (Nunc) were coated with 2 μg/ml capture antibody (eBioscience Inc., San Diego, CA, USA) and stored at 4°C, overnight. All incubations were at room temperature with shaking, and plates were washed with Tris-buffered saline (TBS) (pH 7.4, 0.05% Tween-20) between each step. Two hundred μl blocking buffer was added to each well before a 1-hour incubation. TNF-α samples were diluted at 1 : 4 and 1 : 10; TNF-α was added to each well before 2 hours of incubation. Biotin coupled anti-human antibody (eBioscience Inc., San Diego, CA, USA) was diluted in TBS + 1% bovine serum albumin (BSA) to 3 μg/ml and added to each well and subsequently incubated for 1 hour. Diluted ExtrAvidin®-Alkaline Phosphatase (Sigma-Aldrich) was added to each well prior to 30 min incubation. Finally, 100 μl pNPP substrate (Sigma-Aldrich, 1 mg/ml in 1 M buffer, pH 9.8) was added to each well and incubated for 45 min before the plates were read at 405 nm.
2.7.2. Monocyte Chemoattractant Protein-1 (MCP-1)
MCP-1 secretion was analyzed with a quantitative sandwich enzyme-linked immunosorbent assay (Human CCL2, MCP-1, ELISA kit, 88-7399, eBioscience Inc., San Diego, CA, USA) according to the manufacturer's protocol.
2.8. Cell Viability
The cell viability was measured with the thiazolyl blue tetrazolium bromide (MTT) assay [26] in HT29 cells (HTB-38, ATCC). Approximately 1 × 105 cells were seeded in 96-well plates. After 48 hours of incubation, cells were washed with PBS before fresh media was added. To each well, 100 μl of extracts (final concentration of 50 μg), cell control, or deoxycholic acid (DCA, 500 μM as a positive control) was added. The plates were incubated for 24 hours, washed with PBS, and then 15 μl MTT (M2128, Sigma-Aldrich) was added to each well prior to 4 hours of incubating. One hundred μl of solubilizing buffer (2-propanol, hydrogen chloride, and Triton-x-100) was finally added to each well, and the plates were incubated for 4 hours before read at 570 nm.
2.9. Bio-Plex® Multiplex System (Bio Rad)
This system enables detection and quantification of multiple analyses in a single sample volume. Three customized express plates (6-plex, 96-well flat bottom) preblended from human cytokine group 1 (TNF-α, MCP-1, IL-6, interleukin-10 (10), interferon gamma (IFN-γ), and RANTES) were used in this study. The assay was performed according to the manufacturer's protocol (Bio-Rad Laboratories Inc., Hercules, California, USA). These assays were performed after long-term storage (>4 years at −20°C) of the extracts to investigate whether the anti-inflammatory effects were stable through storage.
2.10. Thin-Layer Chromatography (TLC)
Thin-layer chromatography was used to classify lipid classes. One μl (25 mg/ml in dichloromethane (DCM)) of the oil samples and 5 μl (1 mg/ml in DCM) extracts were analyzed on HP-TLC silica 60 plates (Merck Millipore, Billerica, Massachusetts, USA) in a solvent system of heptane:diethyl ether:acetic acid (70 : 30 : 1 v/v/v) to separate sample lipids. The plates were then treated with copper solution (10% cupric sulfate in 8% phosphoric acid), air dried for 10 minutes, and finally put in a cold oven and heated to 170°C. The standards used were 16-1A and 18-5A (Nu-Checkprep, Elysian, USA).
2.11. Statistical Methods
All of the statistical analyses were performed using IBM SPSS Statistics for Macintosh (released 22.0.0.0, SPSS Inc., Chicago, IL, USA). Values are presented as mean ± SD unless otherwise stated. The significance of differences was evaluated using one-way ANOVA, with Tukey's post hoc test. The difference at the level of p < 0.05 was considered statistically significant.
3. Results
3.1. Extraction Yield
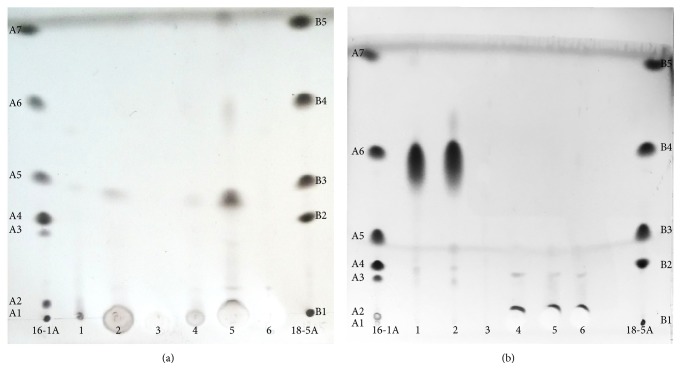
Cold-pressed whale oil was separated into two consecutive samples (CWO-1 and CWO-2) after repeated grinding and centrifugation of the whale blubber. These samples were together with CLO further fractionated into three extracts (EtOAc, BuOH, and H2O) each. There were large differences in extraction yields (expressed as dry matter yields produced from 250 g of the respective oils; mg/g). Dry weights of the extracts from CWO-1 were 0.33 mg/g (EtOAc), 2.07 mg/g (BuOH), and 1.58 mg/g (H2O), and for the CWO-2 extracts, yields were 0.43 mg/g (EtOAc), 0.57 mg/g (BuOH), and 2.17 mg/g (H2O). Contrary to the CWO extracts, the CLO extracts contained only small amounts of water-soluble polar components as reflected in the dry matter yields of 0.60 mg/g (EtOAc), 0.054 mg/g (BuOH), and 0.008 mg/g (H2O). TLC (Figures 1(a) and 1(b)) confirmed that all of the extracts prepared from both CWO and CLO contained very few remaining lipids. It was also evident that the BuOH extracts from CWO-1 and CWO-2 contained very low amounts of residual free fatty acids and no triacylglycerol (TAG), diacylglycerol (DAG), or monoacylglycerol (MAG) (Figure 1(a), lanes 1–6). EtOAc extracts (from CWO-1 and CWO-2) contained some residual phospholipids, whereas the H2O extracts contained no lipids. All the extracts from CLO (EtOAc, BuOH, and H2O; Figure 1(b), lanes 4–6) contained trace amounts of lipids in the form of MAG and DAG, but no TAG. The whale blubber oil and cod liver oil both contained almost exclusively TAG (Figure 1(b), lanes 1 and 2). It is noteworthy that five times more extracts than oils were applied onto the TLC plates.
Figure 1.
HP-TLC of oils (prior to extraction) and extracts. Fatty acid standards: 16-1A containing phospholipids (A1), monoacylglycerol (A2), diacylglycerol (A3), cholesterol (A4), free fatty acids (A5), triacylglycerol (A6), and cholesteryl ester (A7). 18-5A containing phospholipids (B1), cholesterol (B2), free fatty acids (B3), triacylglycerol (B4), and cholesteryl ester (B5). (a) Extracts: CWO-1 EtOAc (1), CWO-1 BuOH (2), CWO-1 H2O (3), CWO-2 EtOAc (4), CWO-2 BuOH (5), and CWO-2 H2O (6). (b) Oils before extraction: CLO (1) and CWO (2) empty (3) extracts: CLO-1 H2O (4), CLO-1 BuOH (5), and CLO EtOAc (6). HP-TLC = high performance thin-layer chromatography; CWO = cold-pressed whale oil; CLO = cod liver oil; EtOAc = ethyl acetate; BuOH = butyl alcohol.
3.2. Antioxidative Capacity
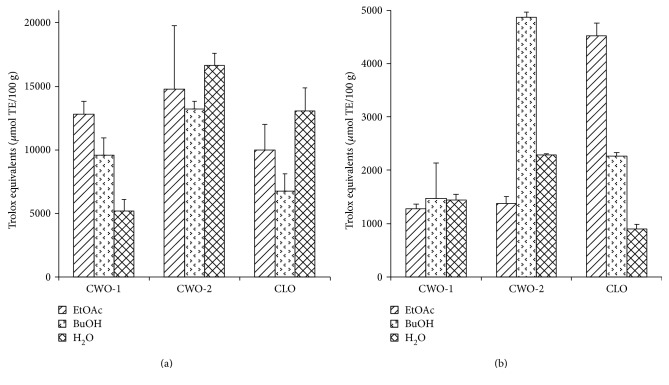
Two methods, ORAC (Figure 2(a)) and FRAP (Figure 2(b)), were applied to determine the AOC of the extracts. In the ORAC assay, it was evident that CWO-2 had high AOC in all three extracts (CWO-2-EtOAc, CWO-2-BuOH, and CWO-2-H2O extracts), and correspondingly, CWO-2 had the highest total AOC. In the FRAP assay, on the other hand, CWO-2-BuOH together with CLO-EtOAc displayed the highest AOC.
Figure 2.
Antioxidative capacity in the extracts. CWO-1, CWO-2, and CLO which were sequentially extracted using EtOAc, BuOH, and water. The results are shown as Trolox equivalents (μmol TE/100 g). (a) ORAC assay with extract concentrations of 0.1 mg/ml. (b) FRAP assay with extract concentrations of 10 mg/ml. CWO = cold-pressed whale oil; CLO = cod liver oil; EtOAc = ethyl acetate; BuOH = butyl alcohol; ORAC = oxygen radical absorbance capacity; FRAP = ferric reducing antioxidant power.
3.3. Anti-Inflammatory Effect on Cytokine Secretion in LPS-Treated THP-1 Cells
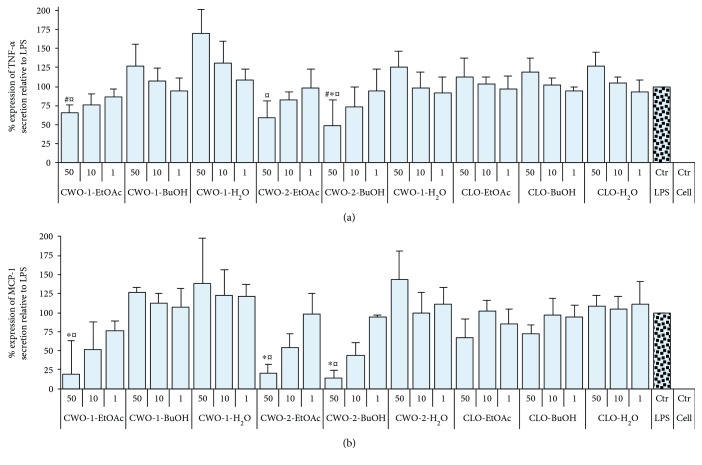
The anti-inflammatory effects of the extracts were studied using LPS-stimulated THP-1 macrophage-like cells. Inhibition of LPS-induced TNF-α and MCP-1 production (Figures 3(a) and 3(b), resp.) was most pronounced for CWO-2-BuOH. The extracts were applied at 3 different concentrations, and a dose-dependent inhibition of TNF-α and MCP-1 secretion was observed for this extract. When applying a dose of 50 μg/ml (CWO-2-BuOH), TNF-α and MCP-1 production were reduced by 50% (p < 0.05) and 85% (p < 0.001), respectively. The extracts CWO-1-EtOAc and CWO-2-EtOAc also inhibited MCP-1 secretion dose dependently, and with a final extract concentration of 50 μg/ml, the MCP-1 secretion was reduced by approximately 80% (p < 0.001, Figure 3(b)). A nonsignificant tendency of the reduction of TNF-α secretion was also observed for CWO-1-EtOAc and CWO-2-EtOAc (Figure 3(a)). None of the extracts affected LPS-induced IL-1β production (results not shown). Negative controls with cell media or vehicle (0.05% DMSO) did not stimulate the release of any of the tested cytokines. Cells incubated with extracts made from CLO were also analyzed; however, no effects were observed on TNF-α (Figure 3(a)), MCP-1 (Figure 3(b)), or IL-1β secretion (results not shown). When comparing the effects of treatment of THP-1 cells with all the extracts from CWO-1 and CWO-2 with the corresponding extracts from CLO, lower LPS-induced TNF-α secretion was observed for CWO-1-EtOAc (p < 0.05) and CWO-2-BuOH (p < 0.005) relative to treatment with CLO-BuOH (50 μg/ml for all extracts, Figure 3(a)). The levels of LPS-induced TNF-α release (p < 0.05) after treatment of the THP-1 cells with 50 μg/ml of CWO-1-EtOAc, CWO-2-EtOAc, or CWO-2-BuOH were lower compared to LPS-induced TNF-α production after treatment with 50 μg/ml CLO-H2O (Figure 3(a)). There was a similar pattern for MCP-1, as LPS-induced MCP-1 release from THP-1 cells treated with extracts (at 50 μg/ml) from CWO-1-EtOAc, CWO-2-EtOAc, or CWO-2-BuOH was different (p < 0.001) compared to treatment with 50 μg/ml of CLO-H2O extract. The cell viability was tested using the MTT assay, and none of the CWO-1 or CWO-2 extracts affected the cell viability (Figure 4()).
Figure 3.
ELISA results for relative response of TNF-α and MCP-1 in LPS-treated THP-1 cells. Each extract was tested at three different concentrations (50 μg/ml, 10 μg/ml, and 1 μg/ml), and the results are presented as mean with SD displayed as positive bars (n = 3). (a) TNF-α secretion relative to control. ∗p < 0.05 compared to LPS control. #p < 0.05 compared to CLO-BuOH 50 μg/ml. ¤p < 0.05 compared to CLO-H2O 50 μg/ml. (b) MCP-1 secretion relative to control. ∗p < 0.001 compared to LPS control. ¤p < 0.001 compared to CLO-H2O 50 μg/ml. TNF-α = tumor necrosis factor-alpha; MCP-1 = monocyte chemoattractant protein-1; LPS = lipopolysaccharide; CWO = cold-pressed whale oil; CLO = cod liver oil; EtOAc = ethyl acetate; BuOH = butyl alcohol.
Figure 4.
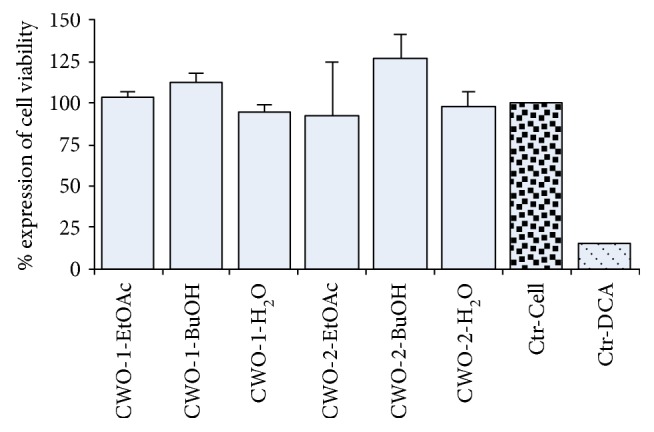
MTT results for cell viability of CWO-1 and CWO-2 extracts. Each extract was tested at 50 μg/ml, and the results are presented as mean with SD displayed as positive bars (n = 3). The cell viability was calculated relative to the cell control (cell media), and 500 μM DCA was included as a positive control. MTT = thiazolyl blue tetrazolium bromide; CWO = cold-pressed whale oil; EtOAc = ethyl acetate; BuOH = butyl alcohol; DCA = deoxycholic acid.
3.4. Effect of Storage on Anti-Inflammatory Activity of the Extracts
The effects of the extracts after long-term storage (>4 years at −20°C) on LPS-induced production of TNF-α, MCP-1, IL-6, IL-10, and RANTES were also assessed (Table 1). CWO-2-BuOH (50 μg/ml) inhibited MCP-1 production in LPS-treated THP-1 cells with more than 60% (p < 0.05). In cells treated with CWO-1-EtOAc, both LPS-induced TNF-α and MCP-1 were reduced, whereas secretion of anti-inflammatory IL-10 was increased in cells treated with CWO-2-EtOAc (p = 0.054, Table 1). Secretion of LPS-induced RANTES was not affected by treatment with any of the extracts (Table 1). CWO-2-BuOH (50 μg/ml) inhibited IL-6 production (p = 0.058) compared to CLO-BuOH (50 μg/ml), and CWO-2-EtOAc (50 μg/ml) increased IL-10 production (p = 0.077) compared to CLO-EtOAc (50 μg/ml, Table 1).
Table 1.
Cytokine responses in LPS-induced THP-1 cells treated with different extracts result in relative % compared to LPS control. ∗p < 0.05 compared to LPS control, ∗∗p = 0.054 compared to LPS control and p = 0.077 compared to CLO-EtOAc 50 μg/ml, and #p = 0.058 compared to CLO-BuOH 50 μg/ml. TNF-α: tumor necrosis factor-alpha; MCP-1: monocyte chemoattractant protein-1; IL-6: interleukin-6; IL-10: interleukin-10; RANTES: regulated on activation, normal T cell expressed, and secreted; LPS: lipopolysaccharide; CWO: cold-pressed whale oil; CLO: cod liver oil; EtOAc: ethyl acetate; BuOH: butyl alcohol.
| Extracts | Extract conc. (μg/ml) | IL-6 (% of LPS) | IL-10 (% of LPS) | MCP-1 (% of LPS) | RANTES (% of LPS) | TNF-α (% of LPS) |
|---|---|---|---|---|---|---|
| CWO-1-H2O | 50 | 282 ± 92 | 212 ± 208 | 97 ± 17 | 126 ± 26 | 136 ± 81 |
| 10 | 163 ± 40 | 92 ± 29 | 125 ± 23 | 141 ± 8 | 142 ± 67 | |
| 1 | 120 ± 20 | 99 ± 115 | 115 ± 18 | 128 ± 6 | 111 ± 38 | |
| CWO-1-BuOH | 50 | 117 ± 3 | 102 ± 29 | 91 ± 2 | 110 ± 26 | 92 ± 37 |
| 10 | 60 ± 53 | ND | 68 ± 56 | 91 ± 73 | 78 ± 65 | |
| 1 | 95 ± 17 | 92 ± 21 | 103 ± 15 | 116 ± 2 | 102 ± 39 | |
| CWO-1-EtOAc | 50 | 85 ± 9 | 219 ± 106 | 58 ± 7 | 101 ± 27 | 60 ± 14 |
| 10 | 90 ± 3 | 99 ± 35 | 100 ± 8 | 115 ± 10 | 90 ± 30 | |
| 1 | 106 ± 19 | 115 ± 25 | 117 ± 23 | 117 ± 12 | 104 ± 41 | |
| CWO-2-H2O | 50 | 170 ± 49 | 166 ± 121 | 100 ± 11 | 109 ± 22 | 114 ± 50 |
| 10 | 96 ± 7 | 91 ± 22 | 104 ± 9 | 127 ± 9 | 104 ± 22 | |
| 1 | 99 ± 16 | 95 ± 19 | 102 ± 13 | 137 ± 21 | 102 ± 36 | |
| CWO-2-BuOH | 50 | 21 ± 12# | 160 ± 43 | 37 ± 13∗ | 80 ± 25 | 42 ± 18 |
| 10 | 71 ± 13 | 123 ± 35 | 105 ± 11 | 126 ± 24 | 91 ± 33 | |
| 1 | 91 ± 31 | ND | 108 ± 35 | 112 ± 32 | 103 ± 57 | |
| CWO-2-EtOAc | 50 | 105 ± 34 | 272 ± 133∗∗ | 53 ± 11 | 98 ± 28 | 101 ± 65 |
| 10 | 95 ± 17 | 137 ± 68 | 96 ± 12 | 141 ± 45 | 96 ± 38 | |
| 1 | 106 ± 16 | 112 ± 19 | 110 ± 12 | 135 ± 22 | 107 ± 42 | |
| CLO-H2O | 50 | 141 ± 47 | 145 ± 99 | 91 ± 10 | 99 ± 11 | 89 ± 36 |
| 10 | 112 ± 38 | ND | 99 ± 18 | 115 ± 25 | 97 ± 45 | |
| 1 | 121 ± 44 | ND | 115 ± 24 | 117 ± 24 | 101 ± 49 | |
| CLO-BuOH | 50 | 121 ± 23 | 100 ± 21 | 80 ± 6 | 104 ± 29 | 83 ± 37 |
| 10 | 112 ± 32 | ND | 104 ± 14 | 109 ± 8 | 101 ± 44 | |
| 1 | 108 ± 24 | ND | 103 ± 24 | 133 ± 31 | 110 ± 50 | |
| CLO-EtOAc | 50 | 113 ± 18 | ND | 90 ± 16 | 105 ± 14 | 101 ± 49 |
| 10 | 122 ± 43 | ND | 111 ± 21 | 120 ± 12 | 104 ± 45 | |
| 1 | 101 ± 21 | ND | 110 ± 29 | 115 ± 25 | 100 ± 48 | |
| LPS | Ctr | 100 | 100 | 100 | 100 | 100 |
| Cell | Ctr | 0 | 5 ± 4 | 2 ± 2 | 3 ± 3 | 0 |
| DMSO | Ctr | 0 | 5 ± 5 | 1 ± 1 | 4 ± 3 | 0 |
4. Discussion
Previous intervention studies comparing intake of CWO with intake of CLO indicated that CWO has anti-inflammatory effects not observed after intake of CLO [20, 21]. Epidemiological studies during the 1970s indicated anti-inflammatory and antioxidative effects of ingestion of blubber based on the low incidence of CVD among the indigenous people in Greenland [27, 28]. It is however important to notice that the Inuit consumed mainly fish in addition to meat and blubber from seals and whales. It has also been claimed that the prevalence of CVD was underestimated in this population [29, 30].
In our study, most of the lipids, and thus the fatty acids, were removed prior to in vitro testing to investigate whether blubber contained compounds that could act in synergy or in addition to the anti-inflammatory effects previously ascribed to the LC-n3-PUFA. To prevent the destruction of putative temperature-labile lipophilic antioxidants present in the whale blubber, extracts were prepared from CWO at low temperatures (<40°C). After removal of the most lipophilic parts of the oil samples, extracts with different polarity were prepared using EtOAc, BuOH, and water as extraction solvents. Thin-layer chromatography indicated that the BuOH extracts from CWO samples contained very low amounts of residual free fatty acids and trace amounts of TAG, DAG, or MAG. The CWO-1-EtOAc and CWO-2-EtOAc extracts contained some residual lipids, whereas the H2O extracts contained no lipids. For the extracts prepared from CLO, the TLC analysis showed that all these extracts contained very low amounts of free fatty acids, TAG, DAG, and MAG.
The total AOC observed in the extracts in this work may be considered high compared to other organic materials tested with FRAP and ORAC assays [31, 32]. The high AOC indicates that the tested extracts contain antioxidants that may protect against reactive oxygen species in vivo. ORAC assay is regarded more physiologically relevant than FRAP due to pH and temperature; however, it is important to emphasize that both these assays are simplified methods to measure AOC. These assays measure quite different mechanisms and are not fully comparable, and it was not surprising that the CWO-2-BuOH displayed high reducing power in the FRAP assay and lower ORAC activity. However, the total AOC from each sample shows the same ranking order (CWO-2 > CLO > CWO-1). Another important aspect is the dry matter yield. Being 5-6 times lower in CLO compared to CWO-2 and CWO-1, this results in lower total amount of antioxidants in CLO. Despite the simplicity of the assays used, these results provided the fundament for the investigation of anti-inflammatory effects.
Several of the extracts displayed potent and dose-dependent anti-inflammatory activity demonstrated through the reduction of LPS-induced production of chemokine (MCP-1) and cytokine (TNF-α). The most pronounced inhibition of TNF-α and MCP-1 was observed in cells treated with the CWO-2-BuOH extract, but treatment with CWO-1-EtOH and CWO-2-EtOAc also inhibited TNF-α production. It is possible that the concentrations (50, 10, and 1 μg/ml) used in the present study might have been too low to reveal the full anti-inflammatory potential of the extracts.
All the extracts that inhibited LPS-induced TNF-α also inhibited release of MCP-1. However, the inhibiting effects on LPS-induced MCP-1 were much more pronounced, as MCP-1 levels were reduced 80% to 85% by these extracts. MCP-1 is an important contributor for atherosclerosis, and therefore potent and specific inhibitors of MCP-1 may be attractive drug candidates for the prevention of atherosclerosis. Contrary to the extracts produced from CWO, none of the CLO extracts affected any of the investigated cytokines or chemokines. The levels of secreted IL-1β were unchanged after treatment with all the extracts, apparently due to the different regulation mechanisms for IL-1β compared to the other cytokines and chemokines tested in this study [33].
The nature of many bioprospecting projects involves sample collection at very remote locations, before processing, extracting, and further investigation at different laboratories. This actualized a desire to determine if the putative bioactive compounds were sufficiently robust to be discovered with this kind of approach. The effects of long-term storage (>4 years at −20°C) on the anti-inflammatory activity in our extracts were investigated with a multiplex assay including MCP-1, TNF-α, IL-10, IL-6, RANTES, and IFN-γ. The anti-inflammatory activity was preserved for CWO-2-BuOH reducing MCP-1 secretion compared to LPS control. Interestingly, CWO-2-EtOAc increased the IL-10 production compared to both LPS control and CLO-EtOAc. IL-10 is considered to have anti-inflammatory capacity in vivo and is known to hold a critical role as a feedback regulator of a wide range of immune responses [34]. Secreted RANTES levels were not affected by any of the extracts, while CWO-2-BuOH downregulated the MCP-1 secretion. Different signaling pathways activate the production of RANTES and MCP-1 after LPS/Toll-like receptor 4 stimulation. RANTES belongs to the MyD88-dependent pathway [35, 36] whereas MCP-1 belongs to the MyD88-independent pathway [37]. In this study, virtually all of the hydrophobic heptan-dissolvable lipids were removed during the extraction process to establish anti-inflammatory activities independent of LC-n3-PUFA. Since triacylglycerol has also been found to downregulate MCP-1 expression in THP-1 cells [38], additional effects could be expected if the fatty acids were included in the extracts. During the refining process of commercial CLO, the oils are subjected to high temperature treatment, which may cause degradation and loss of putative anti-inflammatory compounds. This might explain the absence of anti-inflammatory activity in the CLO extracts despite the presence of antioxidative capacities.
5. Conclusion
This study has demonstrated that CWO contains antioxidants and anti-inflammatory activities that are not related to its content of LC-n3-PUFA. This indicates that there are unidentified extractable anti-inflammatory compounds present in whale oil yet to be discovered. To investigate the anti-inflammatory effects further, the putative bioactive components need to be isolated and CWO (still containing the fatty acids) should be evaluated in vivo in chronic inflammatory animal models.
Acknowledgments
This work was supported by MABIT (Project UB0046). The skillful technical assistance of Jan Ole Olsen, Ida Kristine Hansen, Marte Albrigtsen, Reidun Klykken Lie, and Guro Edvindsen is gratefully acknowledged. The authors also thank Professor Jeanette H. Andersen for reading and commenting the manuscript. Finally, the authors are grateful to Ellingsen Seafood AS, Skrova, Norway, for providing freshly frozen minke whale blubber.
Disclosure
The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
Karl-Erik Eilertsen, Svein Kristian Stormo, Mari Johannessen Walquist, and Bjarne Østerud conceived and designed the experiments. Mari Johannessen Walquist, Svein Kristian Stormo, and Ida-Johanne Jensen performed the experiments. Mari Johannessen Walquist and Karl-Erik Eilertsen analyzed the data. Mari Johannessen Walquist and Karl-Erik Eilertsen wrote the paper. All authors have read and approved the final manuscript.
References
- 1.Libby P., Ridker P. M., Hansson G. K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global Status Report on Noncommunicable Diseases 2014. Switzerland: WHO press; 2014. [Google Scholar]
- 3.Khan R., Spagnoli V., Tardif J.-C., L'Allier P. L. Novel anti-inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis. 2015;240:497–509. doi: 10.1016/j.atherosclerosis.2015.04.783. [DOI] [PubMed] [Google Scholar]
- 4.Tabas I., García-Cardeña G., Owens G. K. Recent insights into the cellular biology of atherosclerosis. The Journal of Cell Biology. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis—an inflammatory disease. New England Journal of Medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Ait-Oufella H., Taleb S., Mallat Z., Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 7.Deshmane S. L., Kremlev S., Amini S., Sawaya B. E. Monocyte chemoattractant protein-1 (mcp-1): an overview. Journal of Interferon & Cytokine Research. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKellar G. E., McCarey D. W., Sattar N., McInnes I. B. Role for tnf in atherosclerosis? Lessons from autoimmune disease. Nature Reviews. Cardiology. 2009;6:410–417. doi: 10.1038/nrcardio.2009.57. [DOI] [PubMed] [Google Scholar]
- 9.Qamar A., Rader D. J. Effect of interleukin 1β inhibition in cardiovascular disease. Current Opinion in Lipidology. 2012;23:548–553. doi: 10.1097/MOL.0b013e328359b0a6. [DOI] [PubMed] [Google Scholar]
- 10.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta (BBA)–Molecular Cell Research. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 11.Kromhout D., de Goede J. Update on cardiometabolic health effects of ω-3 fatty acids. Current Opinion in Lipidology. 2014;25:85–90. doi: 10.1097/MOL.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 12.Mori T. A. Omega-3 fatty acids and cardiovascular disease: epidemiology and effects on cardiometabolic risk factors. Food & Function. 2014;5:2004–2019. doi: 10.1039/c4fo00393d. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson T., Khademi H., Moghadasian M. H. The role of marine n-3 fatty acids in improving cardiovascular health: a review. Food & Function. 2013;4:357–365. doi: 10.1039/c2fo30235g. [DOI] [PubMed] [Google Scholar]
- 14.Mangge H., Becker K., Fuchs D., Gostner J. M. Antioxidants, inflammation and cardiovascular disease. World Journal of Cardiology. 2014;6:462–477. doi: 10.4330/wjc.v6.i6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiuru P., D'Auria M. V., Muller C. D., Tammela P., Vuorela H., Yli-Kauhaluoma J. Exploring marine resources for bioactive compounds. Planta Medica. 2014;80:1234–1246. doi: 10.1055/s-0034-1383001. [DOI] [PubMed] [Google Scholar]
- 16.Skaug H. J., Gjosæter H., Haug T., Lindstrøm U., Nilssen K. T. Do minke whales (Balaenoptera acutorostrata) exhibit particular prey preferences? Journal of Northwest Atlantic Fishery Science. 1997;22:91–104. doi: 10.2960/j.v22.a8. [DOI] [Google Scholar]
- 17.Ackman R. G. Marine Biogenic Lipids, Fats and Oils. Vol. 2. Boca Raton, FL, USA: CRC Press; 1989. [Google Scholar]
- 18.Strandberg U., Kakela A., Lydersen C., et al. Stratification, composition, and function of marine mammal blubber: the ecology of fatty acids in marine mammals. Physiological and Biochemical Zoology. 2008;81:473–485. doi: 10.1086/589108. [DOI] [PubMed] [Google Scholar]
- 19.Koopman H. Phylogenetic, ecological, and ontogenetic factors influencing the biochemical structure of the blubber of odontocetes. Marine Biology. 2007;151:277–291. doi: 10.1007/s00227-006-0489-8. [DOI] [Google Scholar]
- 20.Osterud B., Elvevoll E., Barstad H., et al. Effect of marine oils supplementation on coagulation and cellular activation in whole-blood. Lipids. 1995;30:1111–1118. doi: 10.1007/bf02536611. [DOI] [PubMed] [Google Scholar]
- 21.Vognild E., Elvevoll E., Brox J., et al. Effects of dietary marine oils and olive oil on fatty acid composition, platelet membrane fluidity, platelet responses, and serum lipids in healthy humans. Lipids. 1998;33:427–436. doi: 10.1007/s11745-998-0224-8. [DOI] [PubMed] [Google Scholar]
- 22.Orkla Health. Mollers. 2016. May 2017, http://www.mollersomega3.com/product/mollers-tran/ [Google Scholar]
- 23.Davalos A., Gomez-Cordoves C., Bartolome B. Extending applicability of the oxygen radical absorbance capacity (orac-fluorescein) assay. Journal of Agricultural and Food Chemistry. 2004;52:48–54. doi: 10.1021/jf0305231. [DOI] [PubMed] [Google Scholar]
- 24.Benzie I. F. F., Strain J. J. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: the frap assay. Analytical Biochemistry. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (thp-1) International Journal of Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Bang H. O., Dyerberg J., Sinclair H. M. The composition of the eskimo food in north western Greenland. The American Journal of Clinical Nutrition. 1980;33:2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 28.Dyerberg J., Bang H. O., Stoffersen E., Moncada S., Vane J. R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? The Lancet. 1978;312:117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- 29.Bjerregaard P., Young T. K., Hegele R. A. Low incidence of cardiovascular disease among the inuit—what is the evidence? Atherosclerosis. 2003;166:351–357. doi: 10.1016/s0021-9150(02)00364-7. [DOI] [PubMed] [Google Scholar]
- 30.Fodor J. G., Helis E., Yazdekhasti N., Vohnout B. “Fishing” for the origins of the “eskimos and heart disease” story: facts or wishful thinking? The Canadian Journal of Cardiology. 2014;30:864–868. doi: 10.1016/j.cjca.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Halvorsen B. L., Carlsen M. H., Phillips K. M., et al. Content of redox-active compounds (ie, antioxidants) in foods consumed in the united states. The American Journal of Clinical Nutrition. 2006;84:95–135. doi: 10.1093/ajcn/84.1.95. [DOI] [PubMed] [Google Scholar]
- 32.Ninfali P., Mea G., Giorgini S., Rocchi M., Bacchiocca M. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. The British Journal of Nutrition. 2005;93:257–266. doi: 10.1079/bjn20041327. [DOI] [PubMed] [Google Scholar]
- 33.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 34.Saraiva M., O'Garra A. The regulation of il-10 production by immune cells. Nature Reviews. Immunology. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 35.Lin R., Heylbroeck C., Genin P., Pitha P. M., Hiscott J. Essential role of interferon regulatory factor 3 in direct activation of rantes chemokine transcription. Molecular and Cellular Biology. 1999;19:959–966. doi: 10.1128/mcb.19.2.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Y.-C., Yeh W.-C., Ohashi P. S. Lps/tlr4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Hennessy E. J., Parker A. E., O'Neill L. A. J. Targeting toll-like receptors: emerging therapeutics? Nature Reviews. Drug Discovery. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y. S., Sung H. J., Son S. J., et al. Triglyceride (tg) down-regulates expression of mcp-1 and ccr2 in pma-derived thp-1 macrophages. Genes & Genomics. 2013;35:125–130. doi: 10.1007/s13258-013-0092-6. [DOI] [Google Scholar]