Figure 5.
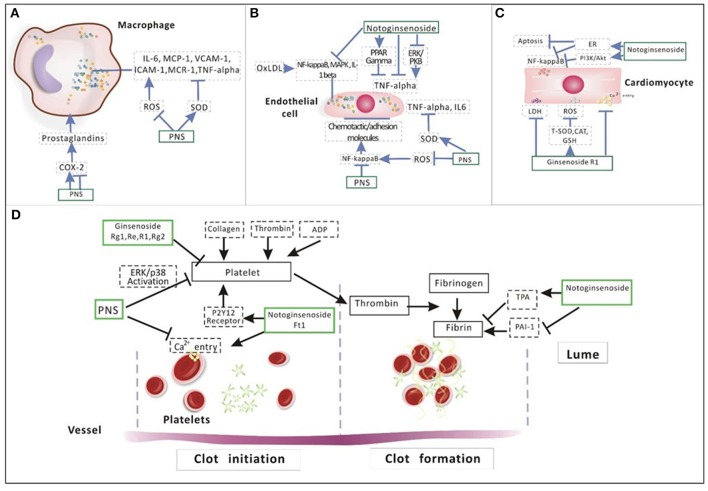
Illustration of the mechanism of PNS on (A) macrophage, (B) endothelial cell, (C) cardiomyocyte, and (D) platelet aggregation. In the process of inflammation among macrophages (A), pro-inflammatory factors such as IL-6, MCP-1, VCAM-1, ICAM-1, MCR-1, and TNF-alpha are regulated by ROS and SOD. Prostaglandins produced by COX-2 is negatively related to phagocytosis. PNS can regulate pro-inflammatory factors by inhibiting ROS and promoting SOD. In addition, PNS also inhibit TNA alpha directly. The activated NF-κB regulates the expression of many atherogenic genes, creating a local inflammatory condition and inducing chemotactic factors and adhesion molecules on the surface of ECs (B). PNS can increase SOD activity by decreasing TNF alpha, IL-6 and ROS generation. Notoginsenoside R1 can suppress inflammatory cytokines production by activating PPAR gamma and by suppressing ERK and PKB, inhibiting TNF-alpha. In addition, NR1 can inhibit NF-κB, MAPK, IL-1 beta and reduce cardiomyocyte apoptosis and inflammation through the activation of ER alpha and PI3K/Akt signaling (C). Ginsenoside Rg1 reduced intracellular ROS and LDH and suppressed the intracellular Ca2+ level by increasing the activity of endogenous antioxidants, including T-SOD, CAT and GSH. About (D), NG have an inhibitory effect on platelet aggregation. The effect of PNS in anti-platelet aggregation is related to the suppression of intracellular calcium mobilization and ERK2/p38 activation. Three main ginsenosides (Rg1, Re, and R1) that exist in PNS also showed anti-platelet activity. Ft1 induced dose-dependent platelet aggregation mediated through P2Y12 receptors. NR1 significantly decreased TNF alpha-induced PAI-1.