Fig. 8.
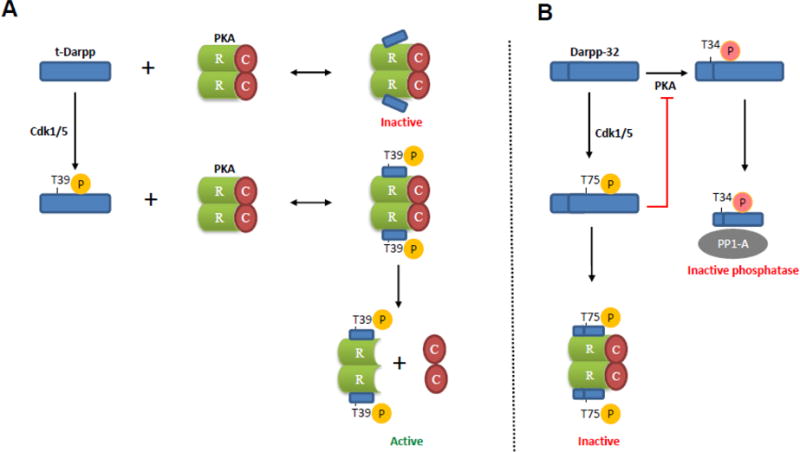
Model illustrating the effects of t-Darpp and Darpp-32 on PKA activity. (A) t-Darpp T39 site is phosphorylated by Cdk1/5 which promotes binding of t-Darpp to PKA regulatory subunit RI (R) and results in release and activation of PKA catalytic subunit (C). The interaction between non-phosphorylated t-Darpp (T39A mutant) and RI may be too weak to promote PKA catalytic subunit release, which would leave the PKA holoenzyme in the inactive state. (B) By contrast, Cdk1/5-mediated phosphorylation of Darpp-32 at T75 converts it into a PKA inhibitor [20]. This inhibition might be mediated by Darpp-32 interaction with PKA regulatory subunit RI, preventing dissociation of RI from the catalytic subunit of PKA. Darpp-32 phosphorylated at another site (T34), mediated by PKA, binds to protein phosphatase-1 (PP1) catalytic subunit, resulting in PP1 inhibition [19]. The inhibition of PP1 results in a net increase in phosphorylation of various downstream substrates.
