Figure 1.
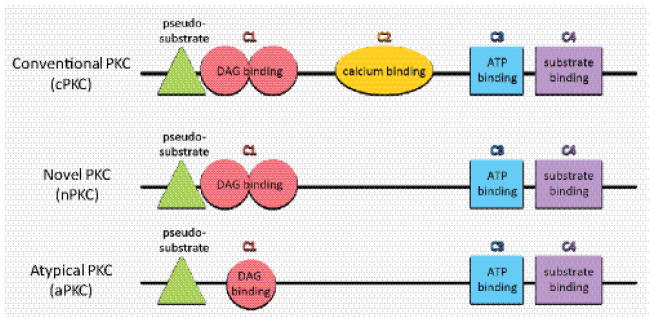
PKC structure is similar across family members, although they have different activation requirements. The inhibitory pseudosubstrate domain and the kinase domain, consisting of C3 and C4, are similar amongst PKCs, but the regulatory domains differ. Conventional PKCs (cPKCs) require DAG and calcium binding, while novel PKCs (nPKCs) require DAG binding, and atypical PKCs (aPKCs) require neither DAG nor calcium binding, although they have a single Cys-rich motif (C1).