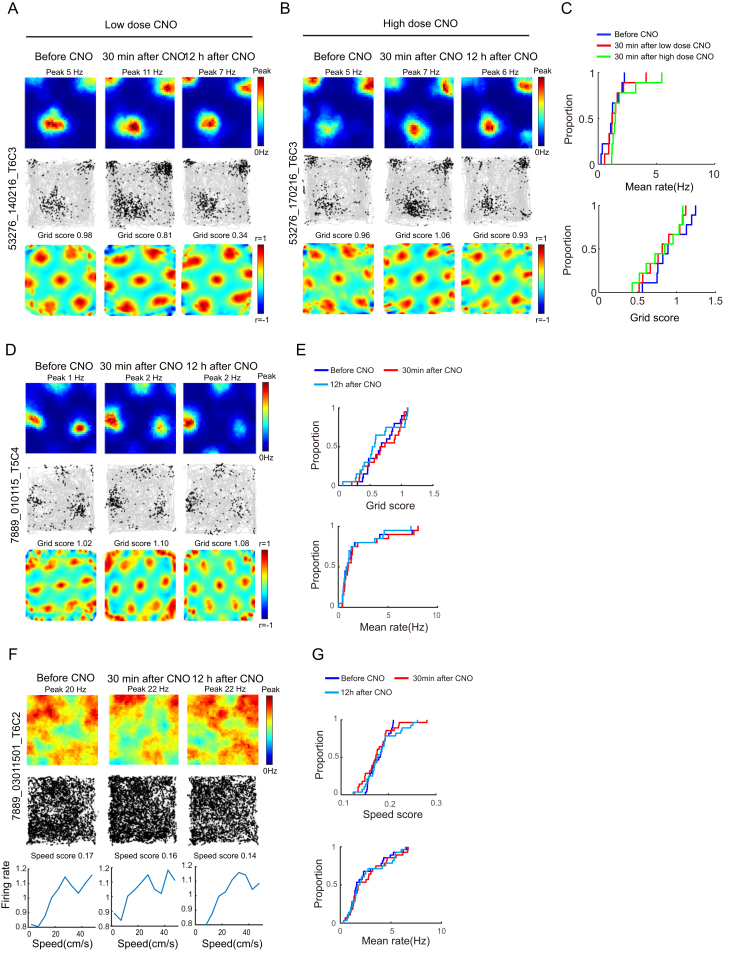
Figure S5.
Grid Pattern Did Not Change after Higher Doses of CNO in the SOM Group nor in Control Animals Not Expressing hM4D, Related to Figures 1, 2, and 3
Effect on grid pattern after higher doses of CNO in the SOM group (A–C) and in control animals not expressing hM4D (D–G).
(A–C) Grid cells were not affected in the SOM group after doubling of the CNO dose. A possible reason for the lack of effect on grid structure might be that the SOM-expressing interneurons were not sufficiently inhibited. The similarity in the number of infected cells and the pronounced change in firing rate speak against this possibility but to test the idea further, we doubled the dose of CNO to 4 mg/kg in one SOM-Cre mouse with 10 grid cells. (A) and (B) show rate maps, trajectory maps, and autocorrelation maps for representative MEC grid cell after a low and a high dose of CNO in an animal with hM4D-mCitrine expression in SOM interneurons (low dose, 2 mg/kg, to the left; high dose, 4 mg/kg, to the right). Data were collected in a 1 m box before CNO, 30 min after CNO, and 12 hr after CNO. Symbols as in Figure 1B. Inactivation of SOM interneurons with the higher dose did not affect the spatial periodicity of grid cells. (C) Cumulative frequency diagrams showing mean firing rate (top) and grid scores (bottom) for grid cells recorded after inactivation of SOM interneurons with low or high dose of CNO. Increasing the dose did not reduce the periodicity of firing in grid cells (grid score before CNO, 0.91 ± 0.076; 30 min after, 0.87 ± 0.082; paired-sample t test, t(9) = 1.31, p = 0.23; 30 min after low dose of CNO in the same mouse, 0.89 ± 0.072, paired-sample t test, t(9) = 1.04, p = 0.33, Figure S5, B and C). As in the experiments with the lower CNO dose, the mean firing rates of the grid cells were increased after the high dose (before, 1.02 ± 0.20 Hz; 30 min after, 1.71 ± 0.31 Hz, paired-sample t test, t(9) = 2.52, p = 0. 04; Figure S5, A and C).
(D–G) Injection of CNO does not affect spatial periodicity of grid cells or speed coding of speed cells in 2 control mice injected with Cre-dependent AAV expressing mCitrine only. hM4D receptors were thus lacking in these mice. (D) Representative MEC grid cell from animal with mCitrine expression in PV interneurons. Data from 1 m box before CNO, 30 min after CNO, and 12 hr after CNO. Symbols as in Figure 1B. (E) Cumulative frequency diagrams showing no change in grid score or mean firing rate of grid cells in the two control mice lacking hM4D. We did not observe any significant change in grid scores after CNO (before, 0.62 ± 0.055; 30 min after, 0.61 ± 0.065; paired-sample t test, t(19) = 0.41, p = 0.68; mean firing rate: before, 1.85 ± 0.51 Hz; 30 min after, 1.89 ± 0.51 Hz; paired-sample t test, t(19) = 1.64, p = 0.12). (F) Representative MEC speed cell from animal with mCitrine expression in PV interneurons. Symbols as in Figure 2A. (G) Cumulative frequency diagrams showing no change in speed score or mean firing rate of speed cells after CNO in the two control mice with only mCitrine in PV cells. There was no detectable change in speed scores after CNO (before, 0.18 ± 0.0037; 30 min after, 0.18 ± 0.0062, paired-sample t test, t(27) = 0.45, p = 0.66).