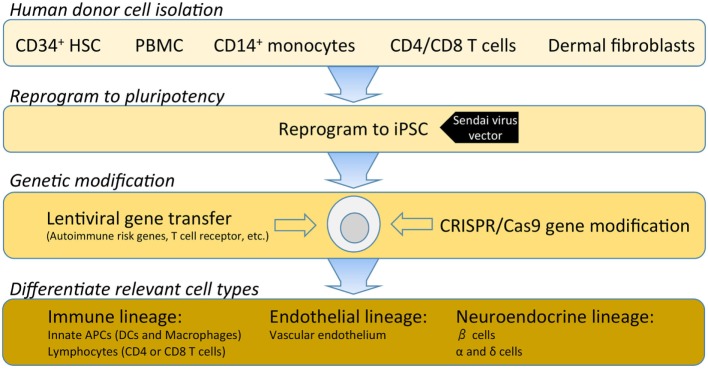
Figure 2.
A hypothetical outline for establishing an isogenic disease-in-a-dish workflow. Induced pluripotent stem cell (iPSC) stable cell lines can be generated from several different somatic cell types depending on specimen availability. Traditionally, dermal fibroblasts from skin biopsy were utilized; however, this is being replaced by less invasive samples such as freshly isolated or cryopreserved peripheral blood mononuclear cells (PBMCs). PBMCs can be enriched for various populations such as CD34+ hematopoietic stem cells, CD14+ monocytes, T cells, or reprogrammed as a bulk population. Where a pre-re-arranged T-cell receptor (TCR) is desired, antigen-specific CD4 or CD8 T cells can be used so that iPSC-derived T cells will clonally express the desired TCR with a naive T-cell phenotype. Several commercial platforms for iPSC reprogramming are currently available. Non-integrating Sendai virus vectors provide a safe and efficient means for iPSC reprogramming of human primary cells. Following reprogramming into iPSC, gene modification enables researchers to investigate disease-associated risk variants and/or over-express or knockdown genes to modulate pathways. Once gene modifications are confirmed, validated protocols for differentiation of immune, endothelial or neuroendocrine lineages are utilized to interrogate the specific effects of each gene variant in several disease-relevant cell types.