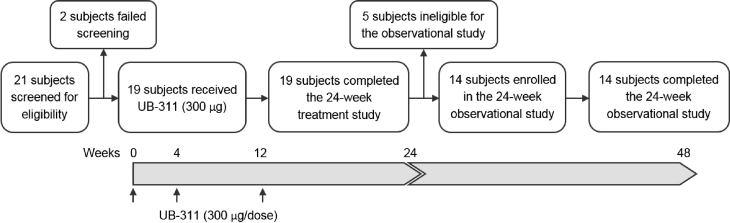
Fig. 1.
Patient disposition and study design. The first-in-human (FIH) clinical trial enrolled 19 patients (50–80 years old) with mild-to-moderate Alzheimer's disease in a 24-week interventional study, and all 19 subjects received three UB-311 vaccine doses by intramuscular injection (300-μg immunogen peptide) at 0, 4, and 12 weeks and completed the treatment study (identifier no.: NCT00965588). After the first five subjects passed the safety evaluation at week 48, the remaining 14 subjects enrolled in and completed a 24-week observational extension study added to the end of the interventional study to monitor long-term safety, tolerability, immunogenicity, and efficacy (identifier no.: NCT01189084).