Fig. 4.
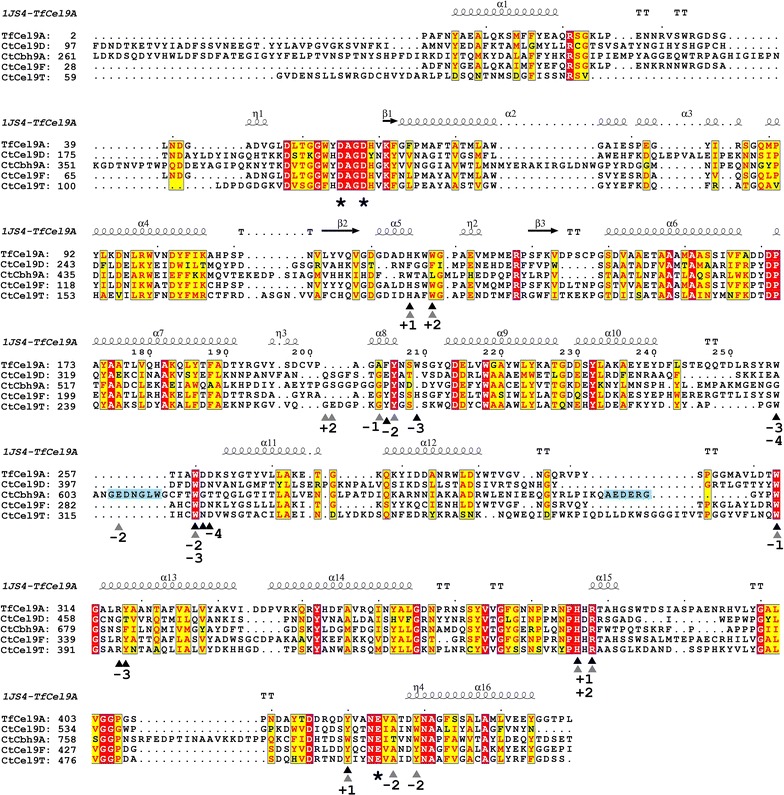
Structure-based multiple sequence alignment of GH9 family catalytic modules of four C. thermocellum cellulases: Cel9D, Cbh9A, Cel9T, and Cel9F. α-Helices (α- and η-helices), β-sheets, and loops in Cbh9A are indicated and numbered above the sequences as squiggles and arrows, respectively. Strict α-turns are indicated with TTT, strict β-turns with TT. The catalytic triad in the active sites is indicated with asterisks. Amino acids of the endoglucanase TfCel9A from Thermobifida fusca known to be involved in substrate-binding [59, 60] are shown as black triangles, those identified from cellobiohydrolase Cbh9A [56] are marked as gray triangles. The numbers below indicate the corresponding cello-oligosaccharide positions reported to interact/bind. Carbohydrate positions + 1 and + 2 are the expected product sites. Loop regions conferring exo-activity of Cbh9A are highlighted in light blue [56]
