Fig. S3.
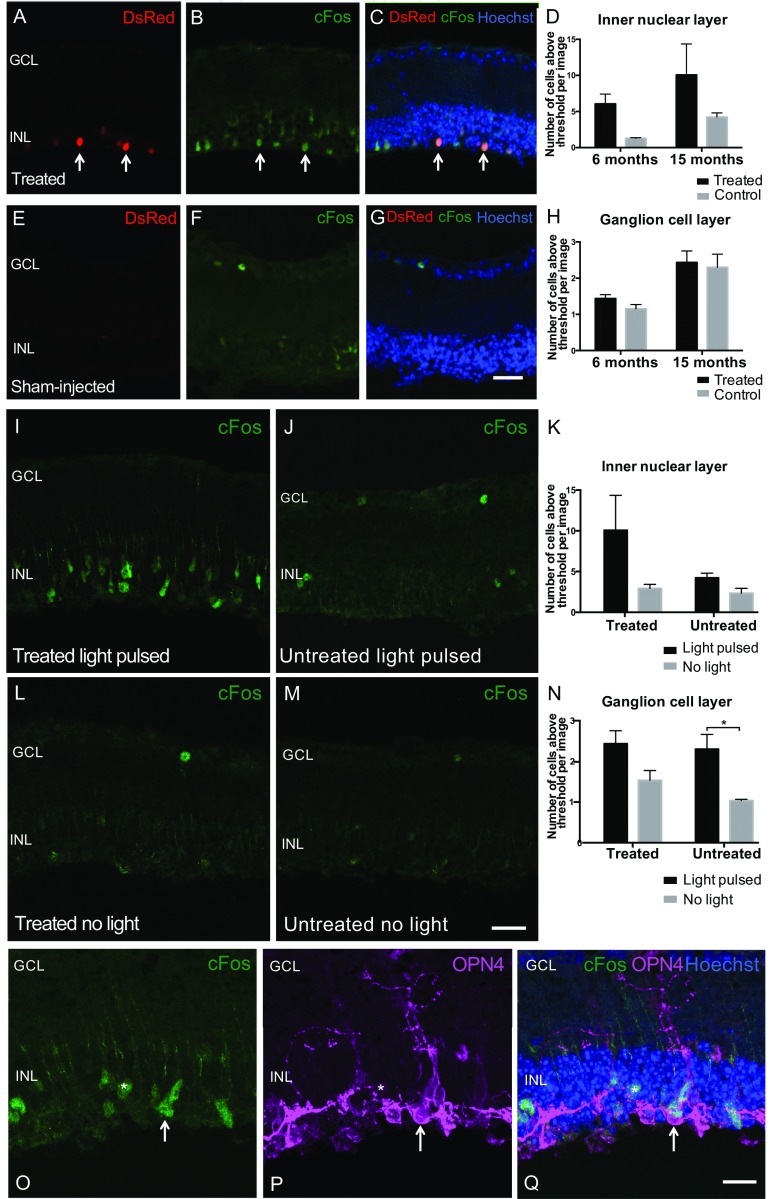
Light-dependent c-Fos up-regulation in inner nuclear layer (INL) cells following subretinal OPN4 vector delivery indicates cell depolarization. To evaluate whether ectopic expression of OPN4 was able to mediate cell depolarization, c-Fos expression was assessed in treated rd1 mice versus age-matched controls at 6 and 15 mo after subretinal vector delivery. Mice were exposed to a 30-min white-light stimulus of 350-lx intensity. Mice were culled after a further 30 min in darkness and retinas collected and examined for c-Fos expression. INL: Areas of vector expression were identified by DsRed fluorescence (red) (A). Antibody staining for c-Fos (green, B) and composite images showing overlay with Hoechst nuclear labeling (blue, C) demonstrate increased c-Fos expression in the INL of treated retina (arrows indicate examples of cells expressing DsRed fluorescence that were labeled with c-Fos antibody). c-Fos expression was not widely seen in the INL of controls following a light pulse (E–G). (Scale bar, 25 μm.) There was a significant effect of treatment (P = 0.0197, two way ANOVA) but not of age (P = 0.103) on the number of c-Fos–positive cells in the INL following light pulsing (D; 6-mo-treated group, n = 303 c-Fos–positive cells, n = 5 retinas; control, n = 50 cells, n = 4 retinas; 15-mo-treated group, n = 302 cells, n = 3 retinas; control, n = 127 cells, n = 3 retinas). Ganglion cell layer (GCL): A few c-Fos–expressing cells were seen in the GCL in each group following light pulsing, but there was no effect of treatment (P = 0.3419) on the number of these cells (H; 6-mo-treated group, n = 72 c-Fos–positive cells, n = 5 retinas; control, n = 45 cells, n = 4 retinas; 15-mo-treated group, n = 73 cells, n = 3 retinas; control, n = 69 cells, n = 3 retinas). Previous work in wild-type mice has shown only 3–5% of ganglion cells express c-Fos in response to light (42) with the remainder of c-Fos–expressing cells in the GCL being amacrine and intrinsically photosensitive ganglion cells (43). The presence of only a small number of c-Fos–positive cells in the GCL of treated mice is consistent with this, and since the activation of amacrine cells is largely inhibitory they may not be depolarized by direct optogenetic activation of inner nuclear cells. To confirm that c-Fos expression was up-regulated by a light stimulus and not by other vector-related effects, retinas from mice subjected to a light pulse (I and J) were compared with those from mice handled in an identical manner but not exposed to light (L and M). There was a significant effect of light on the number of c-Fos–positive cells in the GCL following a light stimulus (N; P = 0.0038, two-way ANOVA; untreated group, P = 0.02, Bonferroni post hoc test), but the overall effect of light did not reach significance examining cells in the INL (K; P = 0.0739). *P < 0.05, Bonferroni post hoc test. To evaluate whether c-Fos–expressing cells in the INL expressed ectopic OPN4, retinas were colabeled with c-Fos antibody (green) and OPN4 (purple) along with Hoechst nuclear stain (blue). This demonstrated c-Fos expression in OPN4-expressing cells (arrow, O–Q). (Scale bar, 25 μm.) A few adjacent c-Fos–positive cells were identified that did not colocalize with OPN4 staining (*) suggestive of cell-to-cell signaling following a light stimulus.