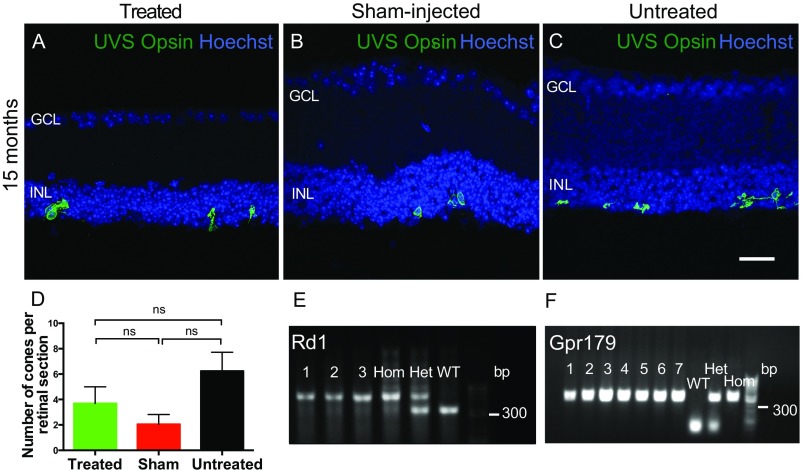
Fig. S6.
Restoration of visual function is not mediated by residual cones. Degeneration of cones in the rd1 model is slower than that of rods, and to assess whether any functional differences seen between groups might be attributed to residual cones, retinal sections were immunolabeled with antibody for UV/S-cone (UVS) opsin (green) in OPN4 vector-treated (A), sham-injected (B), and untreated (C) eyes from mice 15 mo after injection. Images are overlaid with Hoechst labeling of nuclei (blue). Few cones remained in any group, with loss of outer segments and mislocalization of opsin to the cell body, indicating that these cells are unlikely to be functional. There were no significant differences in cone number between groups (D; P = 0.123, one way ANOVA), suggesting that these cells were not responsible for any group differences in behavioral or functional testing. Treated, n = 14; sham-injected, n = 8; untreated, n = 12. ns, nonsignificant. (Scale bar, 25 μm.) All mice were genotyped for the rd1 mutation before use using a duplex PCR protocol (E). Samples from several mice are shown (1–3), with homozygous, heterozygous, and wild-type controls indicating that all mice used were homozygous for the rd1 allele. C3H-derived mouse lines are known to carry a mutation in gpr179, a G-protein receptor required for bipolar cell depolarization in conjunction with the mGluR6 cascade to gate TRPM1 cation channels (26). All mice were genotyped and found to be homozygous for the gpr179 mutation (F). Therefore, residual photoreceptors in these mice would not have been able to drive ON bipolar cell depolarization, or any functional responses via this pathway.