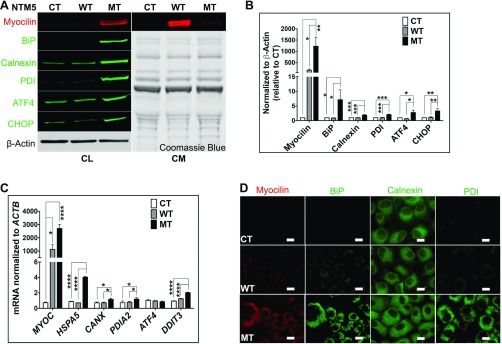
Fig. S1.
Mutant myocilin accumulates inside TM cells. (A) Stably transfected NTM5 cell lines overexpress WT and mutant (MT) forms of human MYOC compared with naïve untransfected control (CT) cells (n = 4). While mutated myocilin accumulates in cells, as demonstrated by analysis of the cell lysate (Left; CL), the WT form is efficiently secreted into the conditioned medium (Right; CM). The accumulation of mutant myocilin causes ER stress, as shown by increased expression of BiP, Calnexin, PDI, ATF4, and CHOP proteins in MT cells. β-actin served as a loading control for cell lysate, and Coomassie blue gel stain was used to test equal loading of secreted proteins in conditioned medium. (B) Densitometry showing significantly higher levels of myocilin, BiP, Calnexin, PDI, ATF4, and CHOP in NTM5 cells overexpressing mutant MYOC (MT) (n = 4) compared with CT or WT cells. (C) qRT-PCR showing overexpression of MYOC mRNA in WT and MT cells and significantly increased expression of ER stress markers mRNA in MT cells (n = 4). (D) An NTM5 cell line overexpressing MT myocilin showing ER accumulation of MYOC (DsRed), BiP (green), Calnexin (green), and PDI (green) compared with CT or NTM5 cells overexpressing WT myocilin. (Scale bar: 100 µm.) Error bars represent SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, one-way ANOVA.