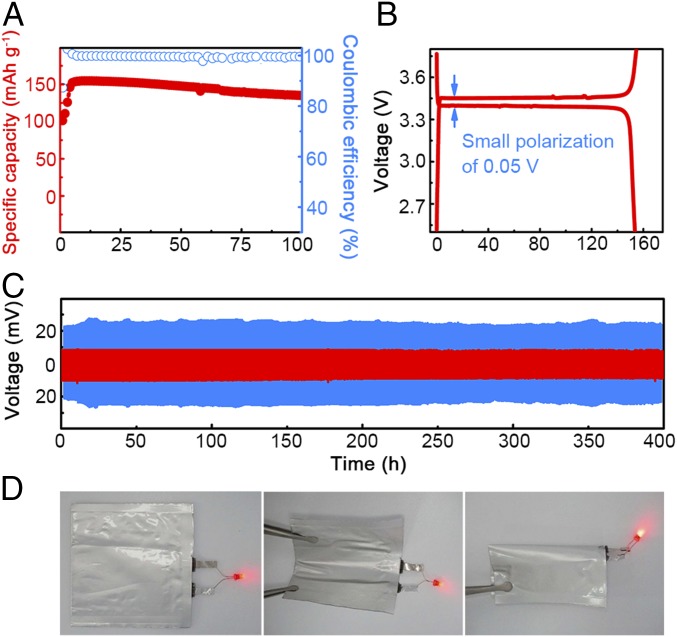
Fig. 4.
Electrochemical cycling performance of the PLL electrolyte. (A and B) Cycling performance (A) and corresponding galvanostatic discharge/charge profile (B) of the all–solid-state LFP | Li metal battery at a rate of 0.1 C and at 60 °C (1.0 C = 180 mA⋅g−1). (C) Voltage profiles of the lithium plating/stripping in a Li–Li symmetrical cell with PLL (60 °C) and routine liquid electrolytes (25 °C) at a current density of 0.10 mA⋅cm−2. (D) An all–solid-state NCM | PLL electrolyte | Li metal pouch cell was assembled to light the LED at both flat and bended states.