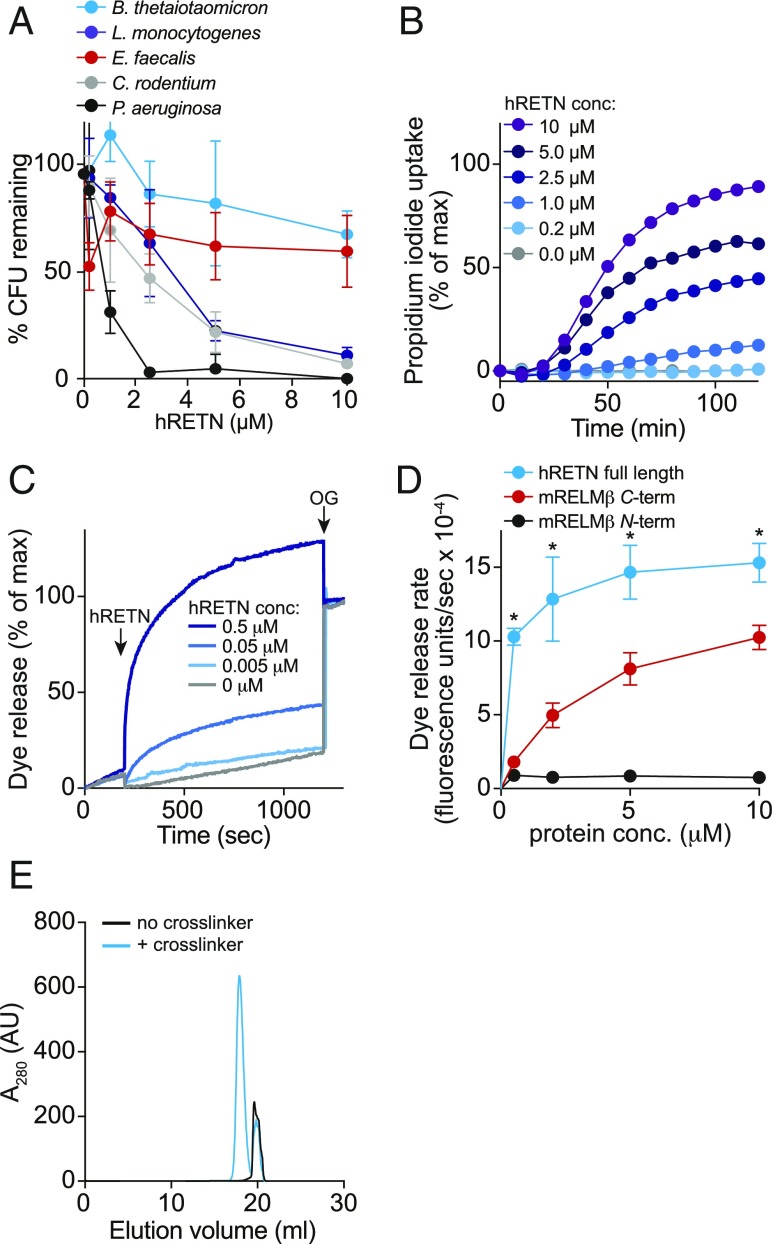
Fig. 4.
Human resistin (hRETN) is a bactericidal protein. (A) Human resistin (hRETN) bactericidal activity. Purified recombinant hRETN was added to midlogarithmic phase bacteria for 2 h, and numbers of surviving bacteria were quantified by dilution plating. Means ± SD are plotted. (B) hRETN permeabilizes bacterial membranes. C. rodentium was treated with increasing concentrations of hRETN, and PI uptake was measured over 2 h. The assay was performed twice and was repeated in triplicate within each experiment. (C) hRETN disrupts carboxyfluorescein (CF)-loaded PC:PS liposomes. Liposomes were treated with increasing concentrations of hRETN, and dye efflux was monitored over time. The 1.0% octyl glucoside (OG) was added at the end to disrupt remaining liposomes. Dye efflux is expressed as a percentage of maximal release by OG. (D) hRETN membrane-disrupting activity is superior to the membrane-disrupting activity of C terminus of mRELMβ. CF-loaded PC:PS liposomes (100 µM) were incubated with varying concentrations of full-length hRETN or the mRELMβ N or C terminus, and initial rates of liposome dye efflux as a function of hRETN concentrations are plotted. Assays were done in triplicate, and means ± SD are shown. (E) hRETN forms a multimeric complex in the presence of liposomes. The 10 μM full-length hRETN was incubated with 100 mM PC:PS liposomes and cross-linked with bis(sulfosuccinimdyl) suberate. Cross-linked complexes were solubilized in detergent and resolved by size exclusion chromatography. Statistics were performed with Student’s t test; *P < 0.05.