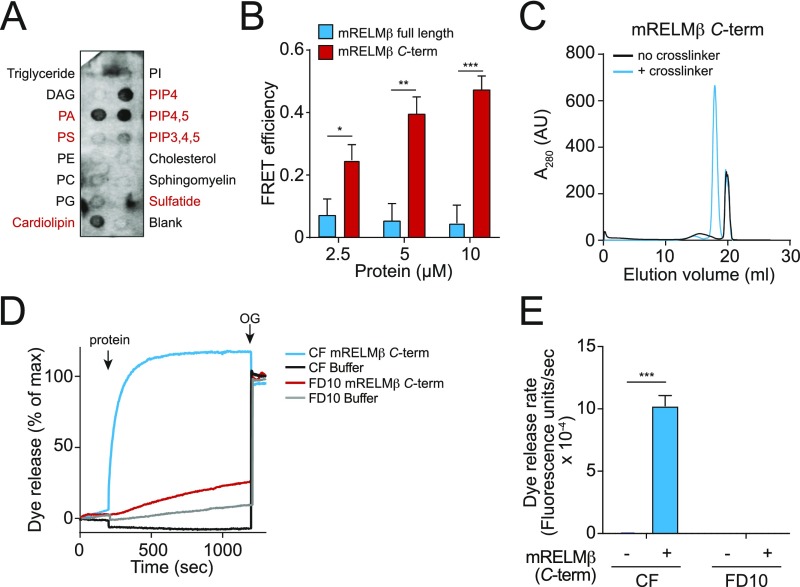
Fig. S4.
Characterization of mRELMβ lipid binding and membrane permeabilization activities. (A) mRELMβ binds to negatively charged lipids (indicated in red). Membranes displaying lipids were incubated with 1 μg/mL mRELMβ, followed by detection with anti-RELMβ antibody. (B) FRET efficiency as a function of mRELMβ full-length and mRELMβ C terminus concentration. Assays were performed in triplicate, and means ± SD are plotted. (C) The mRELMβ C terminus forms a multimer in the presence of liposomes. The mRELMβ C terminus was incubated with 100 mM PC:PS liposomes and cross-linked with bis(sulfosuccinimdyl) suberate. Cross-linked complexes were solubilized in detergent and resolved by size exclusion chromatography. (D) The mRELMβ C terminus forms size-selective pores in liposomes. The 10 μM full-length mRELMβ was added to 100 µM PC:PS liposomes loaded with carboxyfluorescein (CF) (∼10-Å Stokes diameter) or fluorescein isothiocyanate-dextran 10 (FD10) (∼44-Å Stokes diameter), and dye efflux was measured. The 1.0% octyl glucoside (OG) was added toward the end to disrupt remaining liposomes. Dye efflux is expressed as a percentage of maximal release by OG. (E) Means ± SD from three independent replicates of the experiment shown in D. Statistics were performed with Student’s t test; *P < 0.05; **P < 0.01; ***P < 0.001.