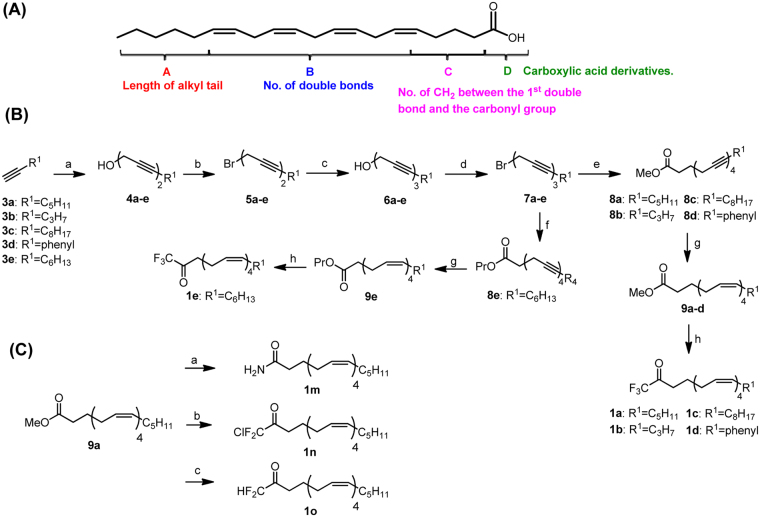
Figure 1.
(A) Functionalities on AA that could be varied. (B) Reagent and conditions: (a) 4-Chloro-2-butyn-1-ol, CuI, NaI, K2CO3, DMF, rt, overnight (b) CBr4, PPh3, CH2Cl2, −40 °C to −20 °C, 1 h (c) Propargyl alcohol, CuI, NaI, K2CO3, DMF, rt, overnight (d) CBr4, PPh3, CH2Cl2, −40 °C to −20 °C, 1 h (e) Methyl 6-hexynoate, CuI, NaI, K2CO3, DMF, rt, overnight (f) Propyl 5-pentynoate, CuI, NaI, K2CO3, DMF, rt, overnight (g) H2, Ni(OAc)2.4H2O, NaBH4, en, 95% EtOH, rt, 2 h (h) i) NaOH, MW 120 °C, 1 h ii) Trifluoroacetic anhydride, Pyridine, CH2Cl2, rt, 2 h (C) (a) NH3, Mg(OMe)2, MeOH, 80 °C, overnight (b) i) NaOH, MW 120 °C, 1 h ii) Chlorodifluoroacetic anhydride, Pyridine, CH2Cl2, rt, overnight (c) i) NaOH, MW 120 °C, 1 h ii) Difluoroacetic anhydride, Pyridine, CH2Cl2, rt, overnight.