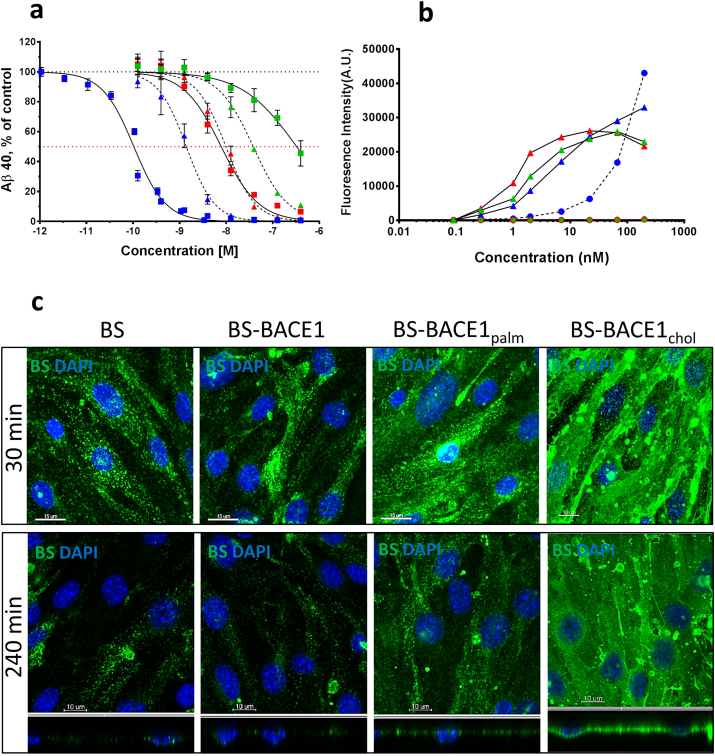
Fig. 4.
Aβ inhibition, TfR binding, and cell uptake of BACE1 peptide inhibitors and BS-BACE1 peptide conjugates in vitro. (a) Aβ production inhibition in HEK293 cells stably transfected with wild-type human APP (APP695) cDNA. Cells were incubated with the different compounds for 16 h (n = 3). Both free and BS-conjugated BACE1 peptide inhibitors blocked Aβ production. Pep#16 BACE1 (green triangle) Pep#15 BACE1palm (red triangle) Pep#14 BACE1chol (blue triangle) BS-BACE1 (green square) BS-BACE1palm (red square) BS-BACE1chol (blue square). The BS-BACE1chol peptide was most potent and is enhanced by the attached to the BS. (b) Cell binding measured by FACS on cells with and without TfR expression. BA/F3 cells (triangles) expressing TfR showed binding of BS-BACE1 (green), BS-BACE1palm (red) and BS-BACE1chol (blue). HEK cells (circle) with no TfR expression showed no binding of BS-BACE1 (green) and BS-BACE1palm (red), but binding of BS-BACE1chol (blue). (c) Uptake and localization of the BS 30 and 240 min after a single dose of 100 nM was assessed in bEnd.3 mouse brain endothelial cells. Unconjugated BS showed time dependent cell uptake, as did BS-BACE1 and BS-BACE1palm. Strong cell surface binding was seen for the BS-BACE1chol construct. X–Y axis (square) and X–Z axis (rectangle).