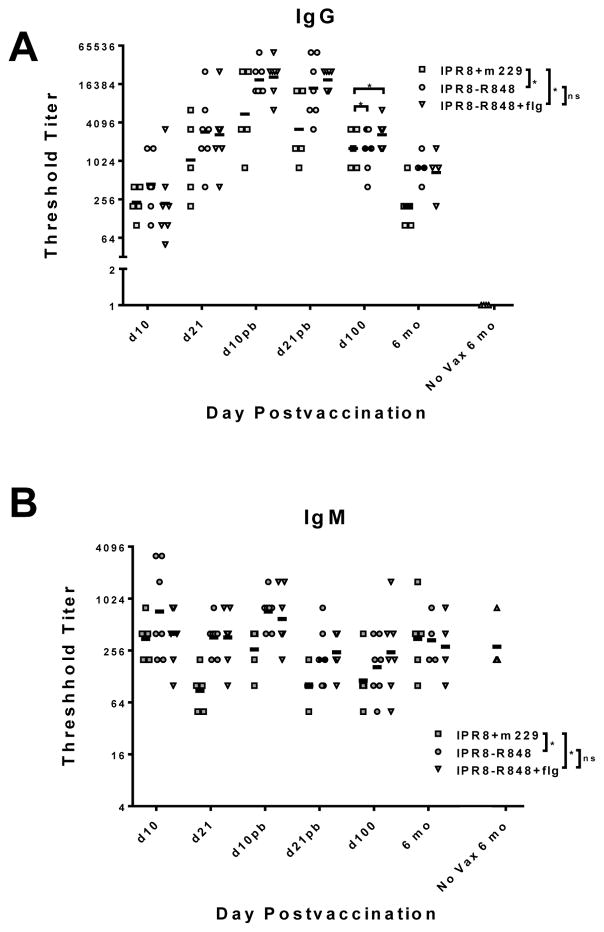
Figure 1. Influenza-specific IgG in infants vaccinated with IPR8-R848, IPR8-R848+flg or IPR8+m229.
PR8-specific IgG (A) or IgM (B) was measured in the plasma of vaccinated (IPR8-R848 and IPR8-R848+flg, IPR8+m229) and non-vaccinated infants. The threshold titer for individual animals and the geometric mean for each group are shown. Infants with no detectable titer are assigned a value of 1 for visualization purposes. For the timepoints through d100 there were 5 animals for the IPR8+m229, and seven animals for the IPR8-R848 and IPR8-R848+flg groups. Five animals were maintained through 6 months for the IPR8+m229 group and 4 animals for the IPR8-R848 and IPR8-R848+flg groups. Significance over the time course was assessed by one-way ANOVA (indicated on the legend). The 6 month timepoint was also individually assessed given our focus on maintenance of the immune response. Influenza-specific IgG but not IgM was significantly increased at this time (indicated over 6 month timepoint data). *p<0.05.