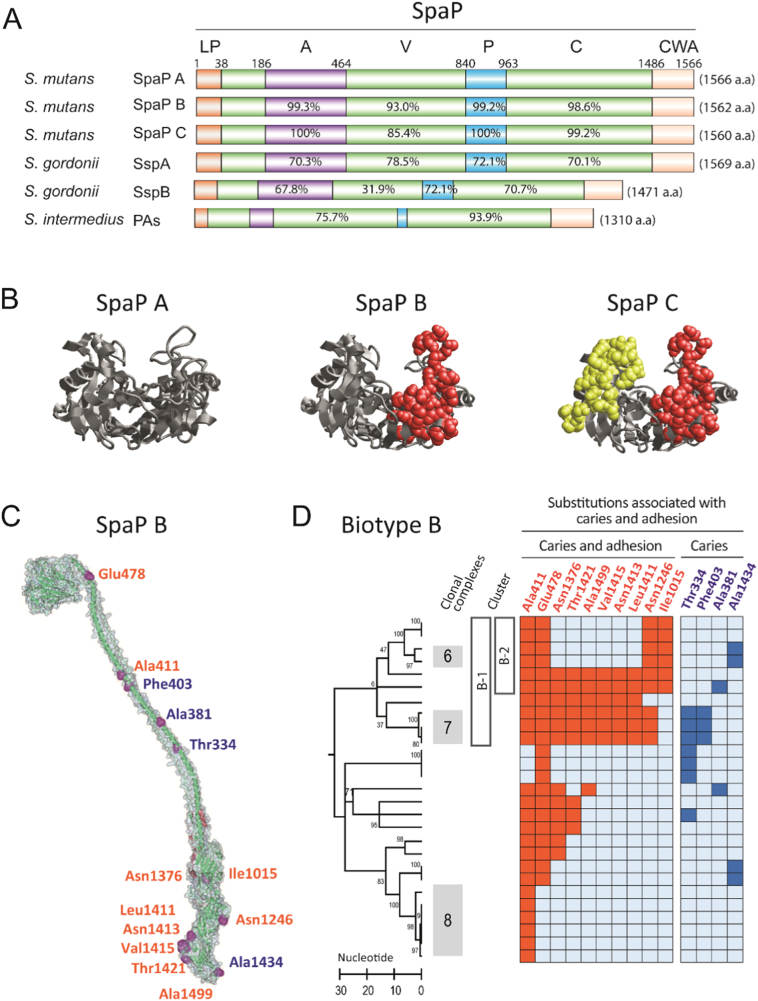
Fig. 4.
Adhesins SpaP A, B and C of different cariogenicity. A) Domain (A, V, P and C) structure and sequence identity of SpaP A, B and C and orthologs in various streptococci (reference SpaP A). The SpaP A, B and C adhesins differ by clustered substitutions in the V-domain and by single substitutions in the A-, P- and C- domains. LP = leading peptide, CWA = cell wall anchoring region. B) The clustered amino acid substitutions specific to SpaP A, B, and C localize on opposite sides of a pocket in the V-domain; A as reference, B and C share 29 substitutions (side chains in red), C holds 32 unique substitutions (side chains in yellow). C) SpaP structure with a tip-localized V-domain, intertwined P and A domains followed by C- and N-terminal complexes. Marked are all substitutions associated positively with caries and adhesion (red) or with caries alone (blue) in SpaP B isolates. D) Amino acid substitutions in SpaP B isolates (marked by a row) that coincide positively with caries and adhesion (to DMBT1 and saliva) or with caries alone upon PLS analyses. The substitutions are enriched in B-1 and B-2 isolates. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)