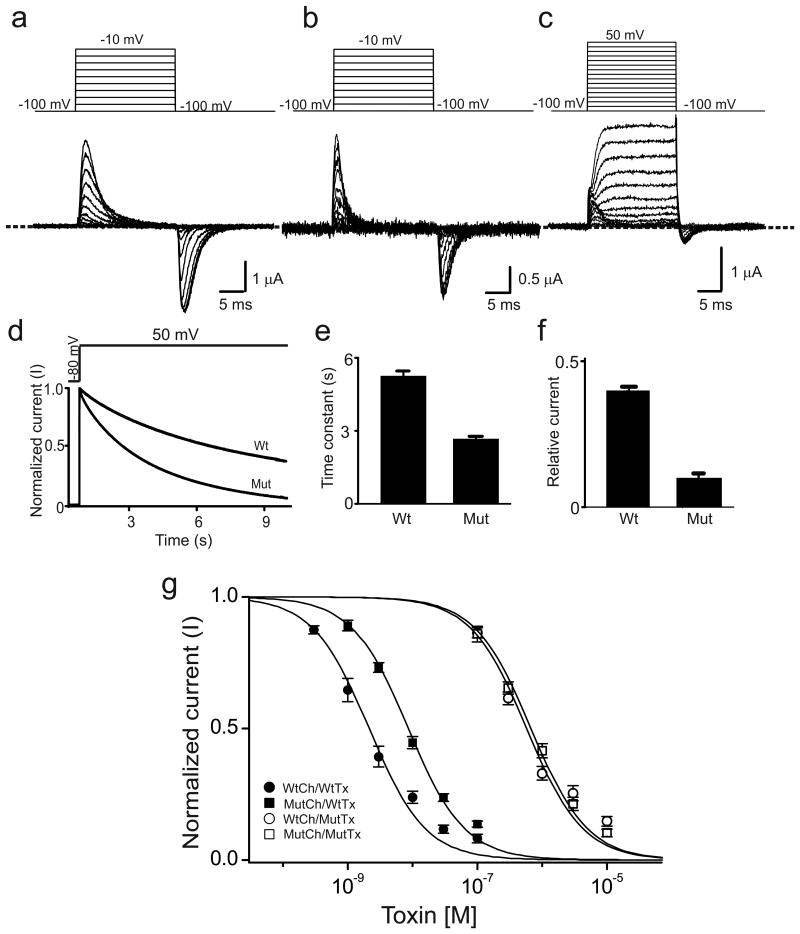
Figure 1.
V478W mutation enhances C-type inactivation in Shaker channels. (a-c) Current traces of homotetrameric V478W (a) or 3/4V478W (b, c) mutant channels. Panel b shows 3/4V478W gating currents whereas panel c shows both gating and ionic currents, where the ionic currents were elicited by stronger depolarization. Currents shown in panels a-c were recorded from three different oocytes in the presence of 100 mM extracellular K+, as membrane voltage was stepped from the -100 mV holding potential to between -90 and -10 mV (a, b) or between -90 and 50 mV (c) in 10 mV increments and back to -100 mV. The dashed line indicates the zero current level. (d) Time courses of wild-type (wt) and 3/4V478W-mutant (mut) channel ionic currents normalized to peak currents, elicited by stepping the voltage from the -80 mV holding potential to 50 mV. (e, f) Mean time constants (e) or currents (normalized to peak currents) remaining at the end of the 10-s voltage pulse (f), obtained from data of the kind shown in panel d; data in e and f are shown as mean ± s.e.m. (n = 16). (g) Normalized current (mean ± s.e.m.; n = 3) of wild-type (circles) or 3/4V478W-mutant (squares) channels (Ch) against concentration of wild-type (closed symbols) or K27M mutant (open symbols) AgTx2 (Tx). Superimposed curves are fits of a monomolecular inhibition model with Kd (mean ± s.e.m.) values of 2.5 ± 0.5 nM for wild-type AgTx2 and wild-type channels, 9.2 ± 1.1 nM for wild-type AgTx2 and mutant channels, 0.58 ± 0.11 μM for mutant AgTx2 and wild-type channels, and 0.67 ± 0.06 μM for mutant AgTx2 and mutant channels.