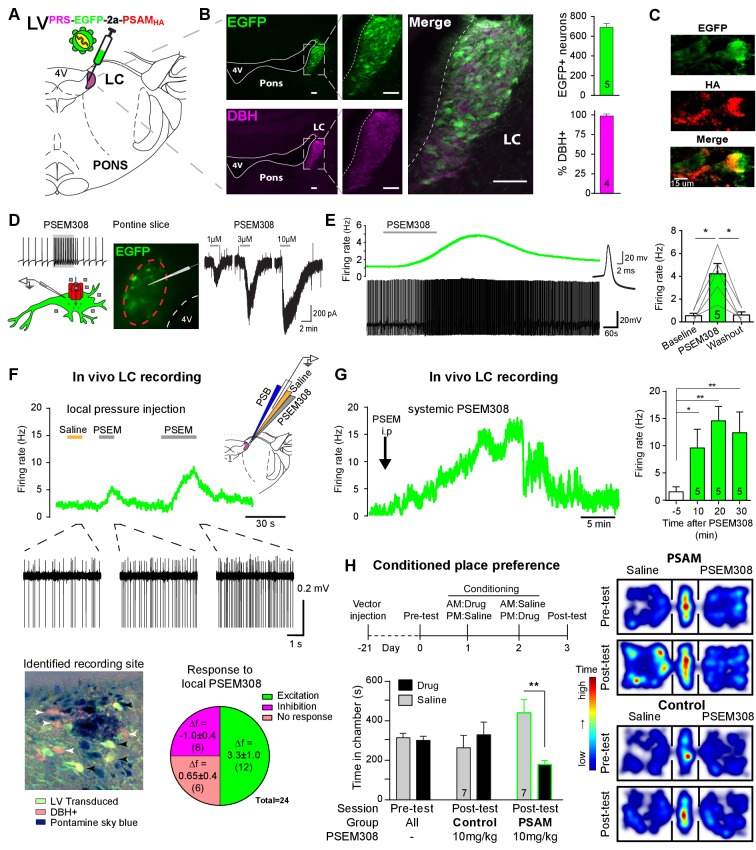
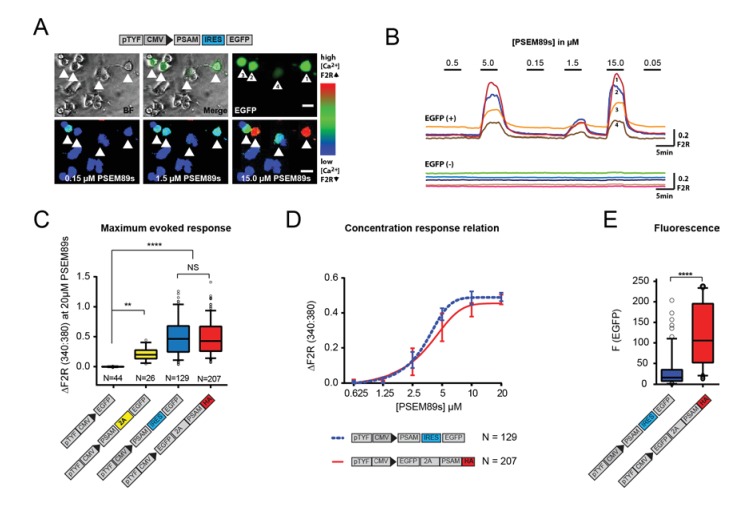
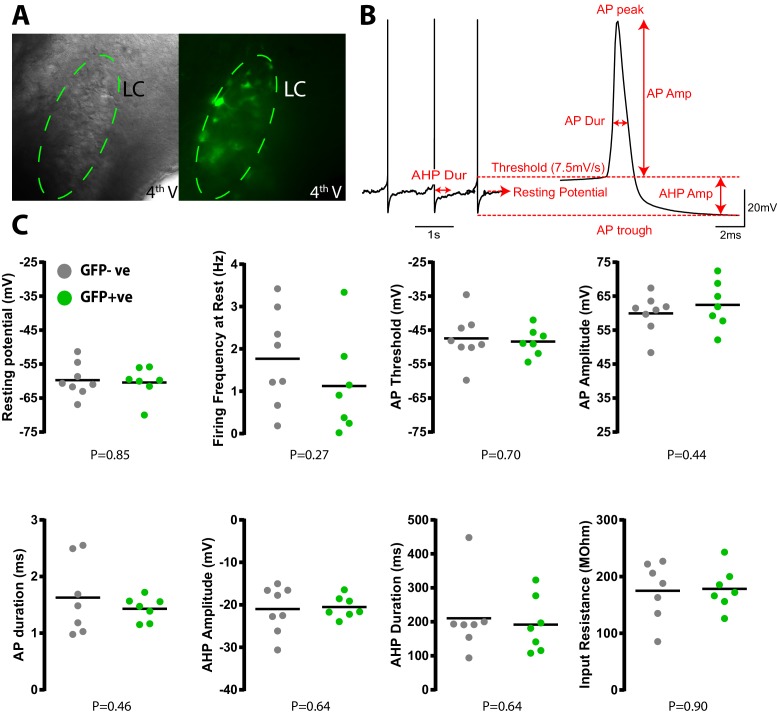
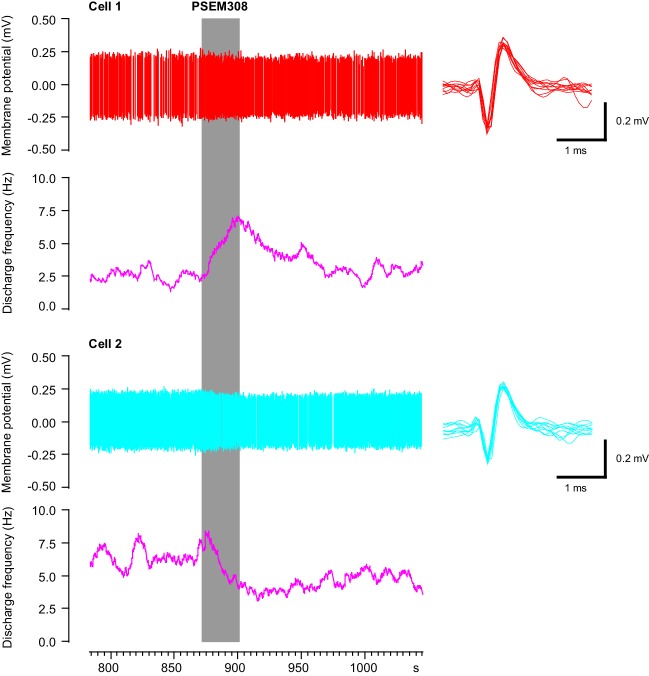
Figure 1. PSAM-mediated chemogenetic activation of LC neurons in vivo.
(A) Strategy using direct stereotaxic injection of lentiviral vector to express the excitatory ionophore PSAM in noradrenergic LC neurons. (B) Selective transduction of LC demonstrated by immunohistochemistry (IHC) for EGFP and dopamine β-hydroxylase (DBH) with 690 EGFP+ neurons per LC of which 98% were DBH+ (scale bar 100 µm). (C) PSAM expression was demonstrated using IHC for the HA tag. (scale bar 15 µm) (D) Schematic of PSEM308-mediated excitation of transduced neurons expressing PSAM. Patch clamp recordings from EGFP+ LC neurons in acute pontine brain slices. Perfusion of PSEM308 evoked concentration-dependent inward currents. (E) PSEM308 (3 µM) increased rate of firing of transduced LC neurons. Inset shows 16 overlaid action potentials. Group data shows increase in firing produced by PSEM308 (3 μM). (F) Extracellular recordings from LC neurons in anaesthetised rats using multi-barrel recording electrodes allowing local pressure ejection of PSEM308/saline/pontamine sky blue (PSB). Traces show graded excitation of an identified LC neuron by PSEM308. Recording sites were subsequently histologically identified by the PSB staining within the LC (transduced cells identified by IHC for EGFP (black arrowheads) and DBH (white arrowheads). The response to local PSEM308 was categorised as excitation or inhibition if it changed firing rate by more than 3 SD from the baseline rate. Application of PSEM308 produced an excitation in 50% of LC neurons. We found a second group of neurons that showed no response, presumably as they were not transduced. A third group showed an inhibition of spontaneous firing in response to local PSEM308 application. (G) Kinetics of the excitatory response to systemic PSEM308 administration (10 mg/kg i.p). (H) Timeline of conditioned place aversion protocol to assess influence of chemogenetic activation of LC neurons on behaviour. In PSAM expressing rats, PSEM308 (10 mg/kg) caused conditioned place aversion but had no effect on control animals. Representative heat maps of rat position in the pre-test and post-test after PSEM308 with bilateral LC transduction with LVPRS-EGFP-2A-PSAM HA or control LVPRS-EGFP All data analysed with repeated measures ANOVA (one or two way as appropriate) with Bonferroni’s post hoc testing (*p<0.05, **p<0.01). (See also Figure 1—figure supplements 1, 2 and 3)