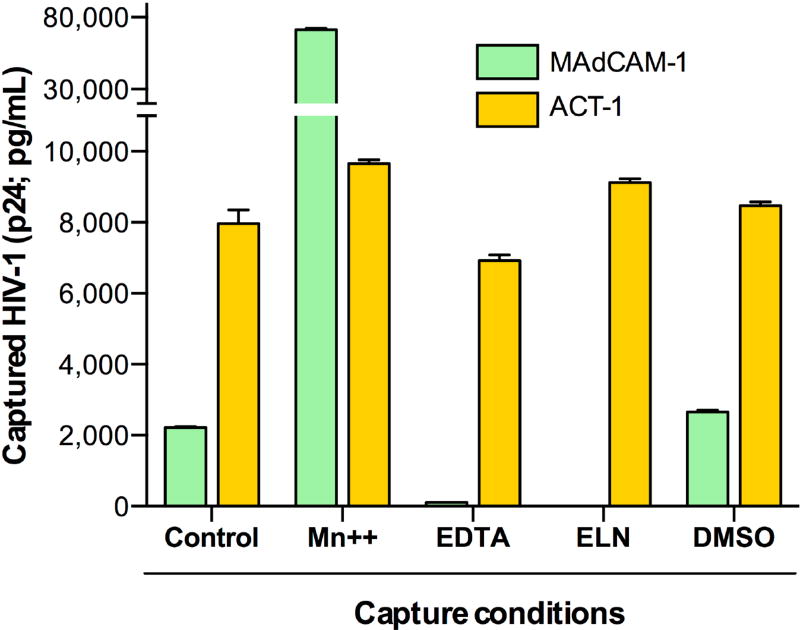
Fig. 3. Virion-incorporated integrin α4β7 is functionally competent.
Virion capture assays were performed on the HIV-1 SF162 α4β7hi viral stock (produced by RA-treated PBMC) in the presence or absence of functional inhibitors or activators of α4β7, compared to the untreated control condition. Capture with recombinant human MAdCAM-1, which selectively binds to the functionally active integrin, was compared to capture with the anti-α4β7 mAb ACT-1, which binds independently of the integrin activation state. The divalent cation Mn++ induces α4β7 to adopt a high-affinity ligand-binding state; the chelating agent EDTA sequesters divalent cations; and the inhibitory peptide mimetic ELN-4757772 (ELN) specifically occludes the MAdCAM-binding site on α4β7, but does not overlap with the ACT-1 epitope. The data represent the mean (±SE) of duplicate samples.