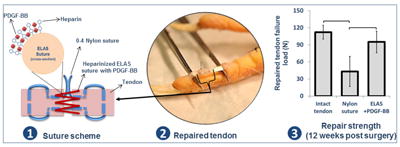
Abstract
Flexor tendon lacerations are traditionally repaired by using non-absorbable monofilament sutures. Recent investigations have explored to improve the healing process by growth factor delivery from the sutures. However, it is difficult to conjugate growth factors to nylon or other synthetic sutures. This study explores the performance of a novel electrochemically aligned collagen suture in a flexor tendon repair model with and without platelet derived growth factor following complete tendon laceration in vivo. Collagen suture was fabricated via electrochemical alignment process. Heparin was covalently bound to electrochemically aligned collagen sutures (ELAS) to facilitate affinity bound delivery of platelet-derived growth factor-BB (PDGF-BB). Complete laceration of the flexor digitorum profundus in the third digit of the foot was performed in 36 skeletally mature White Leghorn chickens. The left foot was used as the positive control. Animals were randomly divided into three groups: control specimens treated with standard nylon suture (n=12), specimens repaired with heparinated ELAS suture without PDGF-BB (n=12) and specimens repaired with heparinated ELAS suture with affinity bound PDGF-BB (n=12). Specimens were harvested at either 4 weeks or 12 weeks following tendon repair. Differences between groups were evaluated by the degree of gross tendon excursion, failure load/stress, stiffness/modulus, absorbed energy at failure, elongation/strain at failure. Quantitative histological scoring was performed to assess cellularity and vascularity. Closed flexion angle measurements demonstrated no significant differences in tendon excursion between the study groups at 4 or 12 weeks. Biomechanical testing showed that the group treated with PDGF-BB bound heparinated ELAS suture had significantly higher stiffness and failure load (p<0.05) at 12-weeks relative to both heparinated ELAS suture and nylon suture. Similarly, the group treated with PDGF-BB bound suture had significantly higher ultimate tensile strength and Young’s modulus (p<0.05) at 12-weeks relative to both ELAS suture and nylon suture. Compared to nylon controls, heparinized ELAS with PDGF-BB improved biomechanics and vascularity during tendon healing by 12-weeks following primary repair. The ability of ELAS to deliver PDGF-BB to the lacerated area of tendon presents investigators with a functional bioinductive platform to improve repair outcomes following flexor tendon repair.
Keywords: Aligned collagen suture, Electrochemical compaction, Flexor tendon repair, PDGF-BB, growth factor delivery
Graphical abstract
1. Introduction
Complete tendon injuries lead to considerable pain and represent a tremendous societal burden accounting for significant disability, high healthcare cost and loss of functionality in the joint distal from the injury [1]. Apposition of the tendon edges using suture remains the gold standard of treatment [2]. However, multiple investigations have demonstrated traditional suture repair to be a slow process requiring prolonged immobilization, resulting in scar formation, decreased mechanical strength and the risk for recurrent tearing secondary to cutting or pulling through tissue [2-4]. As such, an alternative strategy is necessary to expedite tendon healing while ensuring restoration of the tendon biomechanical strength and function.
Growth factors have significant contribution to biological function. In tendon healing process excretion profile of growth factors such as platelet derived growth factor (PDGF-BB), basic fibroblast growth factor (b-FGF), transforming growth factor-β1 (TGF-β1), and vascular endothelial growth factor (VEGF) change significantly over time [5]. Past studies have applied growth factors to the injury site via different approaches [5-7]. It have been shown that in early wound healing stage PDGF-BB and IGF-1 secretions facilitate and accelerate the recruitment of tendon cells to injury site [8, 9] and it has been shown in other works that PDGF-BB [2, 10] and IGF-1[11] enhance tendon cell proliferation, biological activities and collagen synthesis.
Direct injection of growth factor to injury site is an apparently straightforward method of growth factor delivery; however, the short half-life (hours to few days) of the growth factor is the main disadvantage of this approach [12]. Commercially available sutures were coated with biomolecules like gelatin or fibronectin to deliver growth factors. [13-17]. While these methods enhance the precision of the site specificity of delivery to injury site; lack of chemical bonding between coating and the suture surface cause stripping of the coating during the suturing process. Therefore, the bioactive suture concept needs to be improved to retain growth factors for sustained delivery and also to survive suturing-related forces.
Collagen enhances cell adhesion, motility, proliferation and modulate cell fate. To date, pure collagen sutures have not been used to treat lacerated or ruptured flexor tendons. Electrochemical alignment process has been emerged as one of the best method for fabrication of high strength aligned collagen thread which can be utilized as suture [18-20]
In our previous work we demonstrated that functionalizing the aligned collagen thread with heparin molecules provide us with a suture that sustained the delivery of PDGF-BB for up to three weeks in vitro [49]. Prolonging delivery of PDGF-BB resulted in enhanced cell proliferation and collagen synthesis according to results of previous work. Hence, heparinized electrochemically aligned collagen thread has potential to be used as an epitendinous suture for repair of injured tendons.
In this study the performance of a heparinized electrochemically aligned collagen suture with affinity bound PDGF-BB was evaluated for repair of a fully lacerated flexor tendon in a chicken model. Biomechanical and histological outcomes of repaired tendon were compared with heparinized collagen suture without PDGF-BB and traditional synthetic sutures. We hypothesize that use of ELAS sutures with affinity bound PDGF-BB as epitendinous suture will improve the strength of tendon healing, providing a viable and superior repair method.
2. Materials and methods
2.1. Preparation of the collagen suture with affinity bound PDGF-BB
Preparation of heparinized collagen sutures included 4 main steps: 1) fabrication of ELAS sutures with electrochemical compaction method, 2) genipin crosslinking, 3) peracetic acid/ethanol treatment and, 4) EDC/NHS mediated covalent conjugation of heparin before application of the PDGF-BB.
Aligned collagen suture was fabricated by using a custom made automated device as described previously [18]. The device features two parallel electrode wires that are circumferentially wound around a rotating disc. Dialyzed collagen solution (Collagen Solutions Inc., bovine dermis, telocollagen, 3 mg/mL) is applied to the top of the rotating disc between the two electrodes. An electrical current is then applied, resulting in the generation of a pH gradient between the electrodes. Collagen molecules in different pH values acquire different charges (those close to cathode attain negative charge and those close to the anode positive) and under the repulsion forces from the electrodes collagen molecules are pushed and compacted at the isoelectric point. Within less than a minute, liquid collagen transforms into compacted threads with average diameter of 0.11 ± 0.03 mm and collected onto a rotating spool. The biophysical principles of collagen electrokinetics under pH gradients were modeled and discussed in detail in a prior study [21].
Genipin crosslinking imparts mechanical strength to collagen sutures to an extent that is comparable to native tendon as we have demonstrated before [22]. Prior to genipin crosslinking, collagen sutures were incubated at 1× PBS (Fisher scientific) for 6 hours and then kept in isopropanol bath overnight. After these two steps, aligned collagen sutures were crosslinked in genipin solution (0.625 gr in 100 mL of 90% vol. ethanol solution) for 72 hrs [22]. Crosslinked aligned collagen sutures were then washed thoroughly 3 times with deionized water, dried and kept in 4 °C.
Genipin crosslinking decreases the swelling ratio of collagen threads. This limitation was addressed by peracetic acid/ethanol solution (PET) treatment [23]. Collagen sutures were incubated in peracetic acid solution for 4 hrs. PET solution consisted of 2% vol. Peracetic acid solution (Sigma-Aldrich), 200 proof ethanol (Fisher Scientific) and deionized water (volume ratio 2/1/1) [24]. Collagen threads were removed from the solution and rinsed thoroughly in deionized water 3 times for 15 mins each time. Samples were dried and stored at 4 °C.
1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and N-hydroxysuccinimide (NHS) (life technologies) were used to crosslink heparin molecules to aligned collagen sutures. Heparin sodium salt (extracted from porcine intestinal mucosa, Celcus Inc.) was dissolved in EDC-NHS solution (EDC to NHS ratio of 0.72 and pH ≈ 7-8) at concentrations of 10 mg/mL. Collagen sutures were soaked in heparin-crosslinking solution (10 cm of thread in each 2 mL of solution) for 2 hrs. Collagen sutures were then rinsed 3 times with deionized water for 15 mins each, dried completely and stored at 4 °C.
Sulfate groups in heparin provide the collagen threads with negative charge. At neutral pH, net charge of PDGF-BB is positive. Such charge disparity results in affinity binding of the growth factor to sutures, resulting in prolonged delivery of the growth factor as we have shown in vitro in a previous study [23]. The swelling ratio of threads prepared for growth factor delivery was 498 ± 64 wt.% allowing for loading of growth factor. To load the PDGF-BB, sutures were fixed horizontally on a jig and suspended in air. PGDF-BB growth factor solution (concentration of 10 μg/ml ng of the growth factor) was applied on samples with a micropipette. The solution was soaked and taken up completely by the originally dry sutures. Samples were dried and then stored in -20 °C for later use.
2.2. Animal study
The study was approved by the Institutional Animal Care and Use Committee at Case Western Reserve University (protocol No. 2013-0075). We utilized 36 skeletally mature White Leghorn chickens, aged 12 weeks and weighing approximately 1-1.5 kg. Two time-points of 4 weeks and 12 weeks were set in the study. At each time point animals were randomly divided into three groups based on the type of epitendinous suture: control group treated with standard nylon suture (n=6, 6-0 Monocryl suture), group treated with heparinized collagen suture without PDGF-BB (n=6) and group treated with affinity bound PDGF-BB loaded heparinized collagen suture (n=6). The third digit of the right foot was selected to introduce full width laceration while the left foot was left intact as the contralateral positive control. Chickens were housed in individual standard chicken cage and maintained on standard chicken diet, with no limitation of access to food or water.
2.2.1. Experimental Model for Tendon Defects and Repair
On the day of surgery, chickens were anesthetized via isoflurane and maintained on 2-4% isoflurane by inhalation. The skin was sterilized using 3 alternating scrubs of betadine (or chlorhexidine) and isopropanol alcohol prior to incision. Two linear skin incisions were made on the volar aspect of the proximal and distal phalanxes of the third digits of the right foot. In proximal incision flexor tendon sheath was incised and part of flexor digitorum superficialis tendon (FDS) with 1 cm length was cut and removed to expose the flexor digitorium profundus (Fig. 1a-c). In both incisions tendons were sharply transected transversely (Fig. 1e & h). Tendons ends were sutured using a 4-0 synthetic non-absorbable nylon suture (i.e.: Monocryl) core stitch for baseline mechanical integration following with epitendinous suture to keep the ends of tendons close to each other (Fig. 1 f&g and i&j). Operated feet were immobilized using a splint hat extended up to the ankle joint for 2 weeks post operatively. Bandages were renewed at day 7 and the suture area was checked visually to inspect for evidence of infection or necrosis. Six of the operated chickens (2 nylon, 3 heparinized collagen suture, and 1 heparinized collagen suture with affinity bound PDGF-BB) showed signs of mild to severe infection or necrosis which were replaced with new animals.
Figure 1.
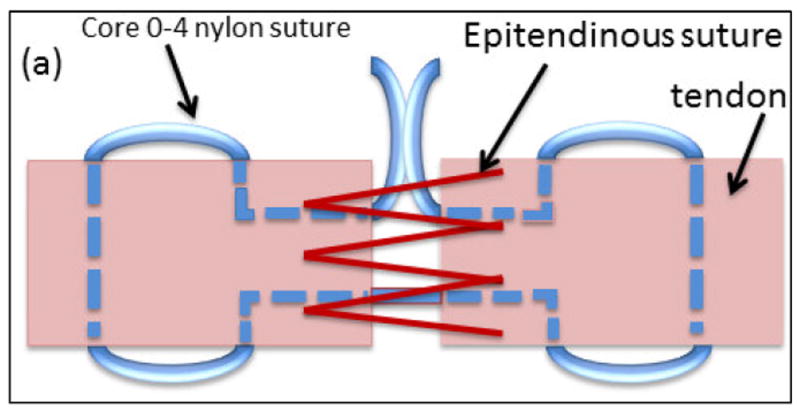
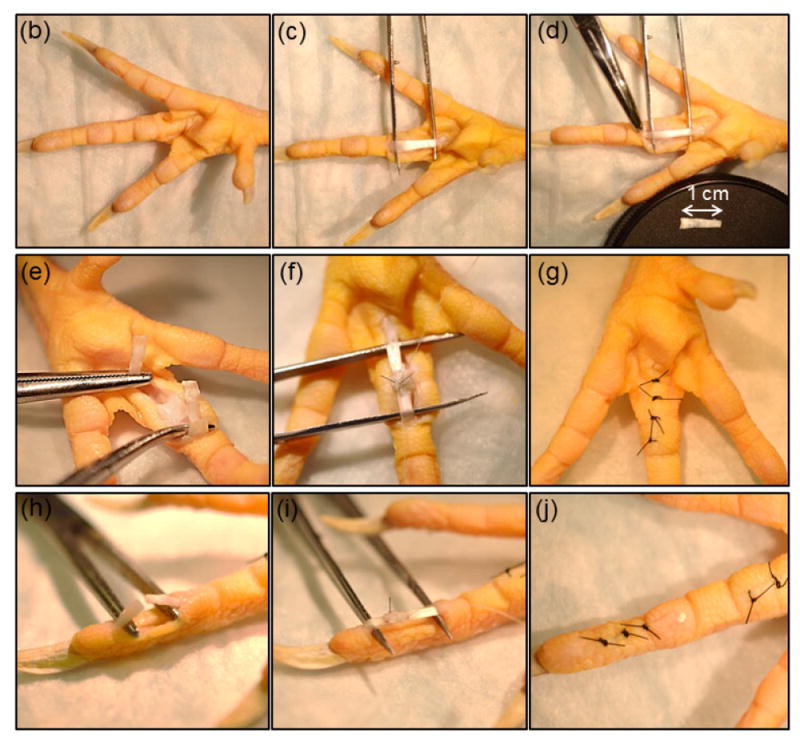
Illustration of suture repair. (a) A core suture (blue suture in the schematic)keeps the two ends of the lacerated tendon together and bears the mechanical load) and an epitendinous suture opposes the two ends of the tendon (red suture). Surgical steps involved incision of the skin on the volar aspect of the proximal phalanx and removing 1 cm length of flexor digitorum superficialis tendon (FDS) to expose the flexor digitorum profundus (b-d), making a complete cut in flexor digitorum profundus tendon at the proximal phalanx, apposition of the two ends of the lacerated tendon by sutures, and closure of the skin (e-g). A similar procedure was followed at the distal phalanx to expose and lacerate the tendon (h-j).
2.2.2. Euthanasia
Animals were anesthetized via isoflurane inhalation and maintained on 2-4% isoflurane then euthanized via overdose of intravenous pentobarbital (3 mL) at 4 and 12 weeks time points. The operated proximal tendons were dissected and harvested for gross morphological analysis and mechanical test while the distal repaired tendons were isolated and fixed in 10% buffered formalin for histological assessment.
2.2.3. Flexion angle measurement
At 12 weeks time point following euthanasia, operated chicken feet were dissected at the ankle and fixed in a custom-made device (Fig.2a) to measure the Flexion angle of the digit. Feet were secured and a constant load of 1.5 N was applied at the proximal end of the tendon. Flexion angles of the proximal, middle and distal phalanxes were measured and compared with intact chicken tendon (positive control) to analyze the degree of mobility and functionality of the treated digit.
Figure 2.
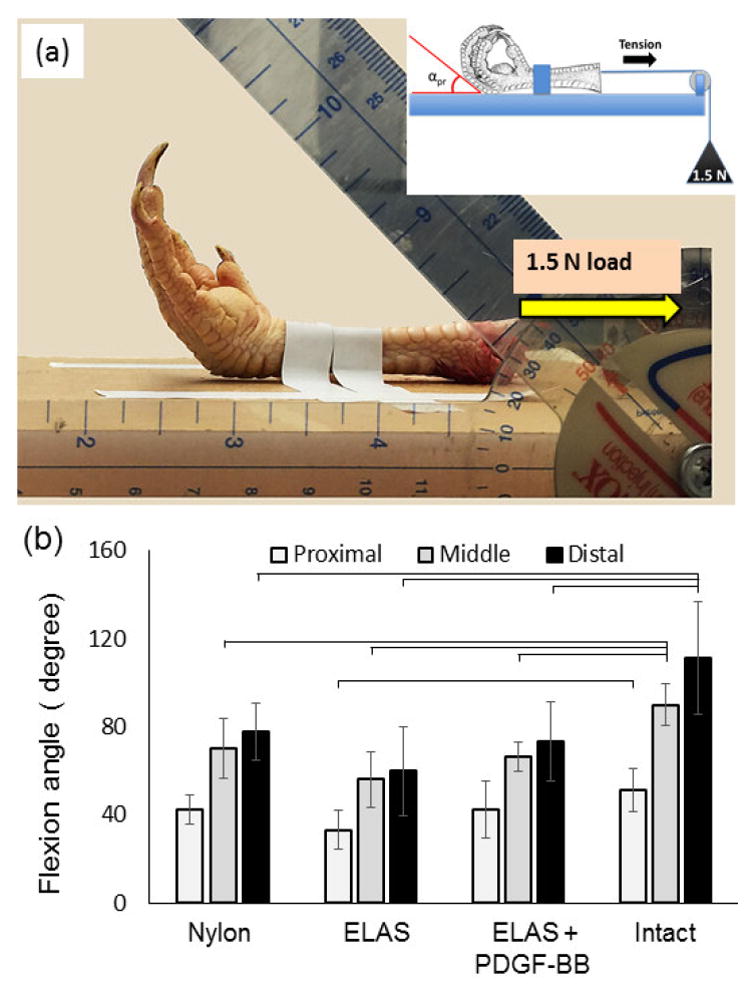
Flexion angle measurement. (a) Chicken claw is fixed on a flat surface and the tendon is pulled at a constant load of 1.5 N applied via a dead-weight. Flexion angles of proximal, middle and distal phalanxes were measured with image processing. (b) All treatment groups (nylon, ELAs and ELAS incorporated PDGF-BB) showed no significant differences (p > 0.05) in flexion angles at 12 weeks following the repair. Flexion angle of intact tendon was found to be significantly higher than all treated groups.
2.2.3.4. Mechanical properties of sutured tendon with ELAS suture
Repaired proximal flexor profundus tendons were isolated and tested in tension (sample size between 5 to 7/group). Load-displacement curves were recorded using a closed loop controlled mechanical testing device (Test Resource 800LE3-2, Test Resources Inc., MN, USA) as described previously [25]. Briefly, samples were kept hydrated with wet gauze soaked in 1×PBS prior to testing. Samples were fixed onto the fixture and subjected to uniaxial tensile loading until failure at a strain rate of 10 mm/min. Failure load, stiffness, elongation, and absorbed energy at failure were calculated from load-displacement data. Repair area was approximated as an ellipse and major and minor axes were measured three times each using a caliper. Cross-sectional area and gage length were used to calculate stress and strain values, respectively, from which ultimate tensile strength, Young’s modulus, strain at failure, and toughness were calculated. Means and standard deviations are reported for all mechanical properties.
2.2.3.5. Histopathological Analysis
After routine preparation steps of fixation, dehydration and block embedding, samples were sectioned in the plane transverse to the longer axis (N= 6 per group per time point). Samples were stained by hematoxylin and eosin (H&E) to visualize cells and masson-trichrome, verhoeff and lectin to enhance the visualization of vascularity.
To visualize the blood vessels sectioned tendonss were stained with Verhoeff and lectin [26-29] stains. For Verhoeff staining sections were rehydrated, stained with working Verhoeff solution (25089-1, Polysciences, Inc.) for 20 minutes, differentiated using 1% tartrazine in 1% citric acid for 3 mins, and counterstained with Van Gieson’s (25089-1, Polysciences Inc.) solution for 3 mins. For lectin staining, sections were gradually hydrated, then blocked for non-specific binding using Carbo-Free blocking solution (SP-5040, Vector Laboratories) prior to addition of biotinylated Sambucus Nigra lectin, isolated from elderberry bark (10 ug/mL, B-1305, Vector Laboratories). Samples were then treated with VECTASTAIN elite ABC (Peroxidase, PK-6100, Vector Laboratories) solution (prepared in accordance with the instruction from the manufacturer) for 30 mins, treated with DAB (MaxTag, DAB-50, Rockland Inc.), and counterstained using Mayer’s hematoxylin.
The total cross sectional area of the tendon diameter was measured by computer software ImageJ (US National Institutes of Health, Bethesda, MD) and reported as means and standard deviations. The cellularity and vascularity were counted for each specimen using computer software (Adobe Photoshop CS-5, CA, USA). Cellularity was quantified by counting the cells in four separate quadrants in fields of view above, below, left, and right of suture at a distance of 5 μm from the edge of the suture. Cell counts were divided by the total area of the field view to obtain the number of cells per mm2. Vascularity was obtained by counting the number of blood vessels over the entire cross sectional area of the tendon. Total vessel counts were divided by the tendon cross sectional area to obtain the blood vessel number per mm2.
Statistical analysis
Data were analyzed with one-way ANOVA followed by Tukey’s pairwise comparison to determine significant differences in Flexion angle, tendon’s cross-sectional area, mechanical properties, vascularity and cellularity between tendons treated with standard nylon suture, tendons treated with ELAS suture with no growth factor and tendons treated with ELAS suture with affinity bound PDGF-BB and tendon with no operation as positive control. Statistical significance was defined as p < 0.05. The quantitative data were expressed as mean and standard deviation.
3. Results
3.1. Flexion angle measurement
The flexion angles of all three phalanges in all treatment groups were not significantly different from each other. All treatment groups had significantly lower flexion at the middle and distal phalanx when compared to intact tendons (p < 0.05). Flexion angles of the proximal phalanx in both nylon and ELAS suture with affinity bound PDGF-BB treated groups were not significantly different (p > 0.05) compared to intact tendon (Fig. 2b).
3.2. Mechanical analysis
All tendons failed at the repair site during biomechanical testing. Failure load for all treatment groups demonstrated no significant differences at 4 weeks following repair (p > 0.05) (Fig. 3a). At 12 weeks, there was a significant increase (P < 0.05) in ultimate failure load of tendons treated with ELAS incorporated PDGF-BB (101.54 ± 45.19 N) compared to 4 weeks time point (40.78 ± 23.83 N). Moreover, the failure load for tendons treated with ELAS incorporated PDGF-BB (101.54 ± 45.19 N) was significantly higher (p < 0.05) than those of treated with nylon suture (43.19 ± 26.45 N) and ELAS without growth factor suture (51.76 ± 31.00 N) at 12 weeks time point. Tendons treated with collagen incorporated PDGF-BB suture had greater stiffness than those of nylon suture and ELAS suture without PDGF-BB at 12 weeks (Fig. 3b). Tendons sutured with ELAS affinity bound PDGF-BB had greater stiffness than that of tendons treated with nylon suture at 4 weeks time point. Tendons treated with ELAS incorporated PDGF-BB had significantly (p<0.05) higher absorbed energy at failure than tendon treated with nylon suture at 12 weeks post surgery. Absorbed energy at failure for tendons treated with ELAS incorporated PDGF-BB demonstrated significant improvement from 4 weeks time point to 12 weeks time point (Fig. 3c) whereas the other treatment groups did not show significant changes. Results of tensile test showed similar elongation at failure for treated tendons in all groups and intact tendon in both 4 and 12 weeks time points (Fig. 3d).
Figure 3.
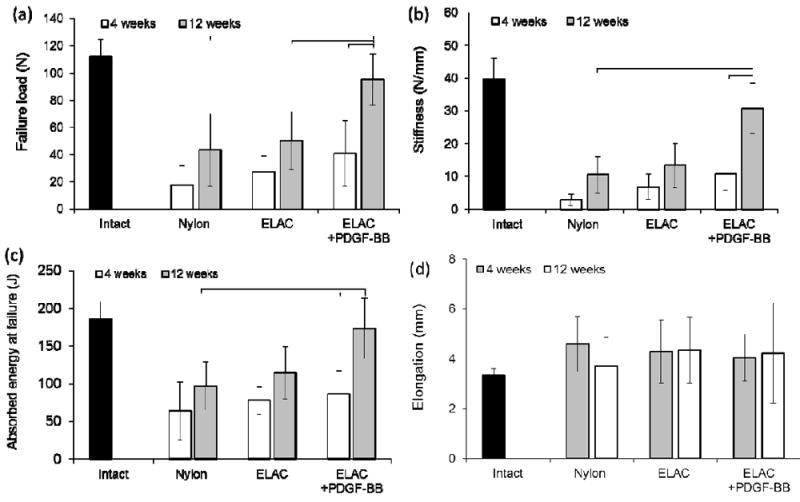
Mechanical properties of tendons repaired with Nylon, ELAS and ELAS incorporated PDGF-BB. Results showed that at 4 weeks post-surgery the failure load for all 3 groups do not differ significantly; however, at 12 weeks time point tendons treated with ELAS affinity bound PDGF-BB has significantly higher (p < 0.05) failure load compared to other groups (a). Moreover, failure load of the treated tendon with ELAS incorporated PDGF-BB suture enhanced significantly (p < 0.05) from 4 weeks to 12 weeks time point. The stiffness and absorbed energy at failure of the treated tendons also showed similar behavior with maximum stiffness and absorbed energy at failure for tendon treated tendon with ELAS affinity bound PDGF-BB (b) & (c). On the other hand the elongation at failure of treated tendons do not show significant differences (p> 0.05) between different groups at different time points (d).
Ultimate tensile strength, Young’s modulus, toughness, and strain at break showed similar trends as described for load-displacement data. Ultimate tensile strength of tendons treated with ELAS incorporated PDGF-BB showed a significant increase from 4 weeks to 12 weeks time point (Fig. 4a). At 12 weeks time point the ultimate tensile strength of tendons treated with ELAS incorporated PDGF-BB was significantly higher (p < 0.05) than those of treated with nylon suture and ELAS without growth factor. Young’s modulus and toughness for treated tendons with different suture materials presented similar behavior as ultimate tensile strength (Fig. 4b & 4c). Strain at failure for all treated tendons with nylon, ELAS, and ELAS with affinity bound PDGF-BB sutures were close with no significant differences in both 4 and 12 weeks time points with failure strains in the range of 18.52 ± 6.63 % to 22.10 ± 5.89 % (Fig.4d).
Figure 4.
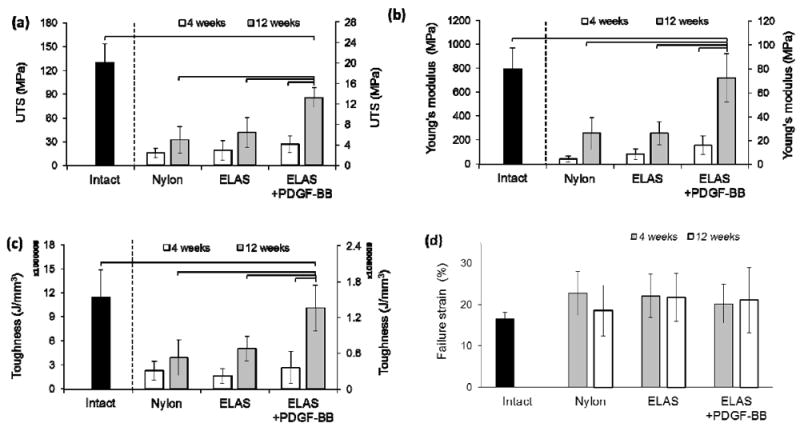
Mechanical properties of tendons repaired with Nylon, ELAS and ELAS incorporated PDGF-BB. Results showed that at 4 weeks post-surgery the ultimate tensile strength for all 3 groups do not differ significantly; however, at 12 weeks time point tendons treated with ELAS affinity bound PDGF-BB has significantly higher (p < 0.05) ultimate tensile strength compared to other groups (a). Moreover, failure ultimate tensile strength of the treated tendon with ELAS incorporated PDGF-BB suture enhanced significantly (p < 0.05) from 4 weeks to 12 weeks time point. The Young’s modulus and toughness of the treated tendons also showed similar behavior with maximum Young’s modulus and toughness for tendon treated tendon with ELAS affinity bound PDGF-BB (b) & (c). On the other hand the strain at failure point of the treated tendons do not show significant differences (p> 0.05) between different groups and time points (d).
3.4. Histopathologic analysis
During harvesting, all repairs were found to be intact without evidence of gap formation. Microscopic examination of H&E stained samples (Fig. 5 a-f) identified no significant difference (p > 0.05) in cellularity of repaired tendon in all three groups at 4 and 12 weeks following repair (Figure 6a).
Figure 5.
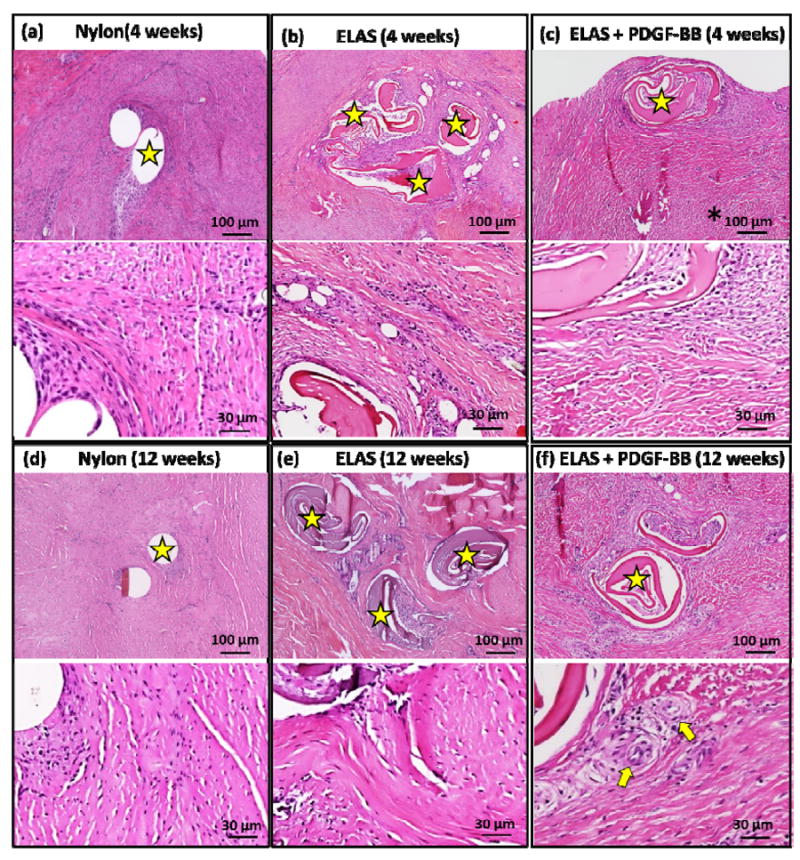
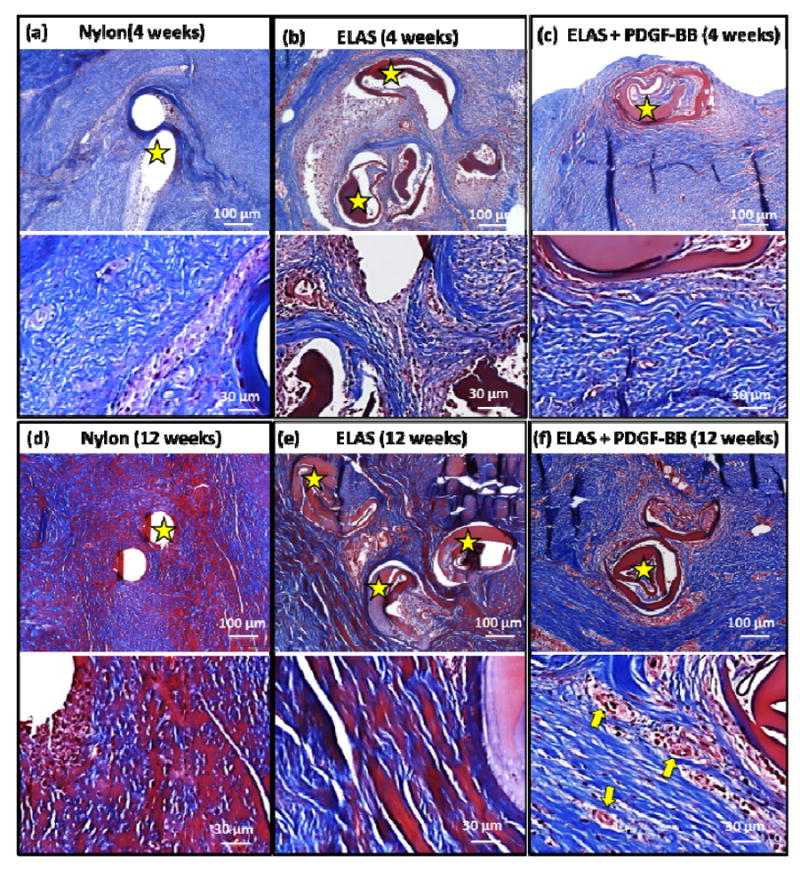
Histological images Hematoxylin & Eosin staining of the tendons treated with Nylon, ELAS, and ELAS with affinity bound PDGF-BB sutures at 4 and 12 weeks post-surgery (a-f). Stars on images show the nylon and collagen sutures in tendon cross-section and arrows show the cross-section of de novo blood vessels.
Figure 6.
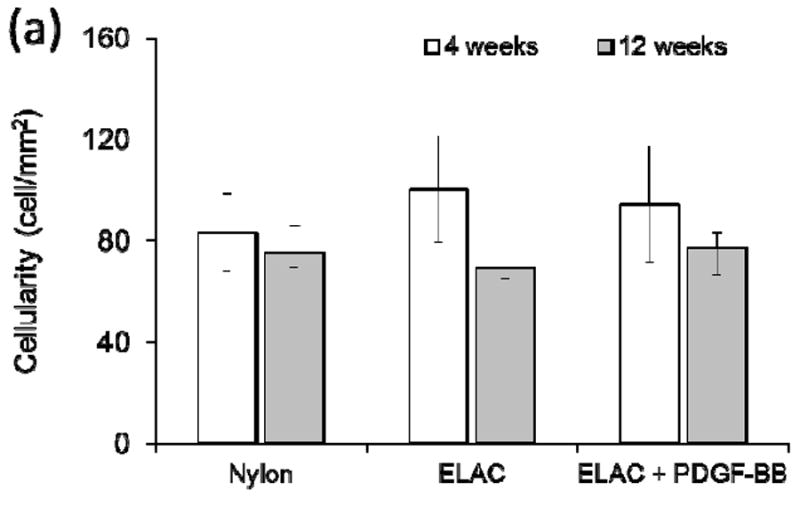
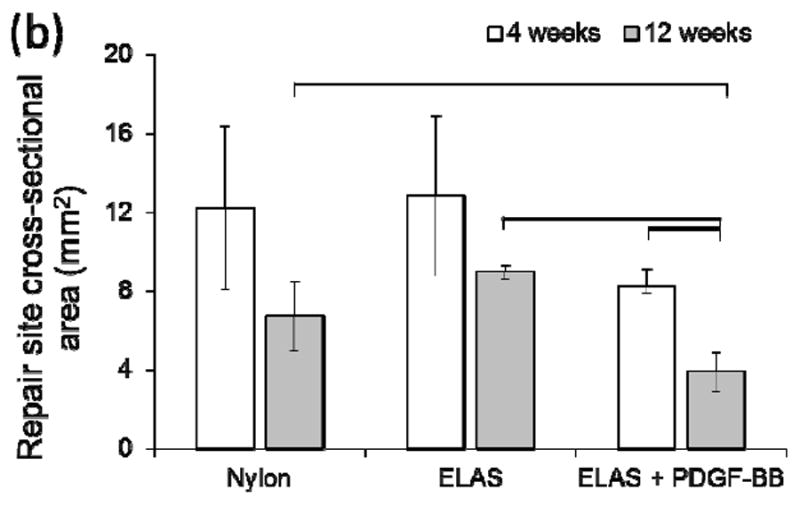
a) Quantification of cellularity of H&E stained sections indicated that there were no significant differences between treatment groups at both time points. (b). Cross sectional area of tendons treated with ELAS incorporated PDGF-BB decreased significantly (p < 0.05) from 4 weeks time point to 12 weeks time point and PDGF-BB treated group’s cross sectional area is significantly smaller than the other two groups at 12 weeks time point.
Cross-sectional areas of the treated tendons at time points of 4 and 12 weeks were measured from H&E stained histological sections (Fig. 5a-f). While the results demonstrated no significant differences in cellularity of repaired tendons in different groups, comparing the cross sectional area of repair site of the treated tendons demonstrated significant decline (p < 0.05) in cross sectional areas of tendons treated with ELAS incorporated PDGF-BB from 4 weeks to 12 weeks time point (Figure 6b). Furthermore, cross sectional areas of treated tendons at repair site for tendons treated with collagen suture with affinity bound PDGF-BB at 12 weeks time point (3.91 ± 1.00 mm) was significantly lower (p < 0.05) than cross-sectional areas in other two treated groups (8.99 ± 0.36 mm for collagen suture treated samples and 6.72 ± 1.78 mm in nylon suture treated samples) (Fig. 6b).
Vascularity of repaired tendons at 4 and 12 week time points was visualized and quantified from masson-trichrome stained samples (Fig.7 a-f). Results demonstrated significant increase (p < 0.05) at 12 weeks time points when compared to 4 weeks time point in each given group (Fig. 7g). At each time point the group treated with collagen suture with affinity bound PDGF-BB had significantly higher (p < 0.05) vascularity compared to the two other groups. Samples at 12 weeks time point were stained with more specific stains of verhoeff, and lectin to have a better visualization of vascularity in tendon cross-sections (Fig. 8). In verhoeff stained samples blood vessels cross-sectioned stained dark blue-black and in lectin stained sections blood vessels appeared as brown.
Figure 7.
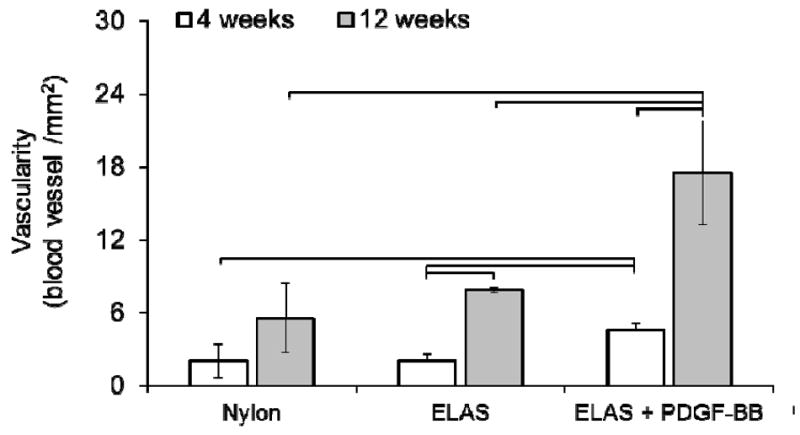
Masson-trichrome stained sections from treatment groups at 4 and 12 weeks post-surgery (a-f). High magnification microscopic examination of Masson-trichrome stained samples demonstrated that ELAS+PDGF-BB group had higher vascularity compared to two other groups treated with nylon suture and collagen suture without growth factor. Yellow arrows indicate the blood vessels in sample treated with collagen suture with affinity bound PDGF-BB. Stars in images show the nylon and collagen sutures in tendon cross-sections and arrows show the cross-section of de novo blood vessels. Quantitative results (g) demonstrated that samples treated with ELAS incorporated PDGF-BB suture demonstrated significantly (p<0.05) higher vascularity at 12 weeks post-surgery compared to tendons treated with nylon and ELAS sutures
Figure 8.
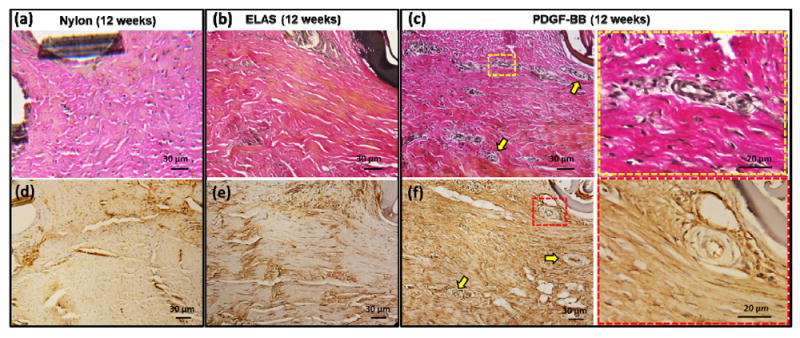
Visualization of the vascularity in repaired tendons at 12 weeks post-surgery time point with specific verhoeff (a-c) and lectin (d-f) staining. Verhoeff stained the blood vessels dark blue/black and lectin stained blood vessel brown. Blood vessels are indicated in images with yellow arrows. Last column of images demonstrates the high magnification of the area indicate in part (c) and (f) with square.
4. Discussion
This study demonstrated that PDGF-BB delivery via heparinated collagen sutures improves the biomechanics of lacerated tendon repair significantly. Quantitative histological assessment indicated that this improvement occurred in parallel with increased vascularity in PDGF treated groups. Concomitant with these changes was a decreasing tendon cross-sectional area at the laceration site at 12 weeks post-surgery, suggesting enhanced remodeling at the repair site. The enhancement in the quality of the healing in PDGF-BB delivered group was also reflected in significantly greater failure stress than the other two groups.
Tendon excursion, as measured by closed flexion angle measurements, demonstrated no significant differences between groups treated with collagen suture with or without PDGF-BB relative to nylon suture. One of the common complications resulting in loss of function following tendon repair is the formation of adhesions within the digital sheath that occurs as a result of the inherent inflammatory reaction leading to cell infiltration and granulomatous tissue formation, in addition to a lack of healing activity between the epitenon and paratenon [17, 30-32]. To achieve optimal repair outcomes, the repair environment must be manipulated to alleviate tendon adhesions without inhibiting the normal healing process. While other studies have shown significant increases in range of motion and excursion values in PDGF-BB treated digits [33, 34], our results found no improvement with the use of PDGF-BB impregnated suture at any of the three measured joints when compared to ELAS suture and nylon suture values. The main reason could be due to the splinting of the claws for two weeks post-operatively. Immobilization was required as the early results demonstrated that shorter fixation time results in repair rupture and separation of the tendon ends in repair site. However, previous studies have shown that muscle loading is necessary to maintain functional properties following repair [4]. Decreasing the splinting period can be used to decrease the adhesion and improve the flexion over time. Furthermore, as a part of our surgery protocol, to prevent the adhesion between treated tendon and the flexor digitorum superficialis (FDS) tendon, the FDS tendon was removed. This process can be another reason for the decreased flexion angles in treated groups compared to control intact tendons. Moreover, the flexion angle measurement was performed at 3 months post-surgery time point. It is possible the 3 months is not sufficient to see differences in Flexion angle of tendons treated with nylon, ELAS suture and ELAS suture with affinity bound PDGF-BB.
While the ultimate failure load, stiffness, and absorbed energy at failure for treated tendons with collagen suture with affinity bound PDGF-BB were very close to intact tendons with no significant differences, ultimate tensile strength, Young’s modulus, toughness of the sutured tendon with collagen suture with affinity bound PDGF-BB are significantly lower than intact tendon. The reason for this difference is related to nature tissue repair in body. Healing process in most soft tissues and in this case, tendon, is a process which occurs in three phases. In the first few days blood clots will form and inflammatory reaction will initiate at the site of injury. Second phase will involve proliferation and repair phase during which intrinsic fibroblasts will proliferate and new blood vessels begin to form under the effect of cytokines. In the last stage of remodeling phase, an unorganized bulky callus forms and slowly remodels to a more slender aligned structure over time. The remodeling step may take up to a year to complete which results in an organized aligned structure will less cellularity and vascularity that is close to the morphology of normal tendon tissue. The time points in this study fell in the remodeling phase of healing. Therefore, the tendons in lacerated site were much thicker (bulky callus form) than intact tendon which resulted in significantly lower ultimate tensile strength, Young’s modulus, and toughness. From a functional point of view, it is the mechanical survival of the entire tendon that matters rather than efficiency. That is why load-displacement behavior is a better functional measure than stress. Several in vivo studies showed that the PDGF-BB improves the quality of healing by improving the remodeling of deposited matrix [35, 36]. The results of this study showed that the cross sectional areas of the group treated with ELAS with affinity bound PDGF-BB was the smallest of all groups, implying that PDGF-BB may have enhanced remodeling.
Our histologic evaluations suggested that the tendons sutured with ELAS suture with affinity bound PDGF-BB had a greater number of blood vessels compared to groups treated with ELAS suture alone or nylon suture. PDGF plays a role in the angiogenesis [37-40] and it is also released by endothelial cells at the tip of forming capillaries and serves to mediate pericytes recruitment [38, 41]. PDGF-BB also stimulates pericytes to produce extracellular matrix proteins, including fibronectin, collagen and proteoglycans, necessary for the membrane of capillaries. In addition, PDGF-BB increases expression levels of vascular endothelial growth factor (VEGF) in progenitors of smooth muscle cells and pericytes and stimulates fibroblasts to produce and secrete collagenases, key for cell migration in angiogenesis [42]. PDGF-BB plays an important role in stabilization, maturation, and functionalization of de novo blood vessels by inducing anastomoses and recruiting pericytes. Fibroblasts, endothelial cells and smooth muscle cells express the PDGF β-receptors which has been shown to have a key function in blood vessel stabilization [43, 44]. Therefore, PDGF may be responsible for the reported improvement in the vascularity of healing tendons in our study.
In this study we introduce a collagenous suture which is able to deliver the PDGF-BB in a controlled fashion. PDGF-BB (positively charged) has charged based affinity to the heparinized collagen suture (negatively charged). In the current study, PDGF solution is applied to dried collagen sutures and the entire solution volume is absorbed by the suture, diffusing the growth factor across the entire volume of the suture. Otherwise, PDGF was not applied as a superficial coating that would slough off. As the results of this study demonstrated increase in cellularity and vascularization we can reach to this conclusion that the affinity bound PDGF-BB was not lost to a significant extent as a result of suturing.
A prior study from our group has characterized in vitro delivery of PDGF-BB from heparinized sutures [23]. It was demonstrated that the delivery of the growth factor was extended for up to three weeks by increasing the amount of heparin that is conjugated. The net effect of heparin mediated PDGF delivery was such that cell proliferation and collagen matrix synthesis by cells were greatly improved in comparison to growth factor free group. The outcome of the past in vitro characterization of these sutures encouraged us to conduct this in vivo study. While we did not measure the PDGF-delivery in this in vitro model, the growth factor appears to have been delivered to an extent to create greater cellularity and vascularization along with enhanced mechanical properties at the healing site. Since soluble PDGF-BB degrades very rapidly in vivo, the presence of PDGF-BB in affinity bound form to heparinized collagen sutures appears to have improved and prolonged the signaling role of this growth factor in healing process of the tendon. This manuscript presents the first work with a collagenous suture that can deliver PDGF-BB for improving the tendon healing in vivo.
Statement of significance.
A high strength aligned collagen suture was fabricated via linear electrocompaction and heparinized for prolonged delivery of PDFG-BB. When it was used to suture a complete lacerated flexor tendon in a chicken model controlled release of the PDGF-BB improved the strength of treated tendon after 12 weeks compared to tendon sutured with commercial nylon suture. Furthermore, Collagen suture with affinity bound PDGF-BB enhanced the vascularization and remodeling of lacerated tendon when it compare to synthetic nylon suture. Overall, electrocompacted collagen sutures holds potential to improve repair outcome in flexor tendon surgeries by improving repair strength and stiffness, vascularity, and remodeling via sustained delivery of the PDGF-BB. The bioinductive collagen suture introduces a platform for sustained delivery of other growth factors for a wide-array of applications.
Acknowledgments
The study was funded by the AO Foundation Grant Number S-12-63K. Research reported in this publication was also supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR063701. This investigation was supported by the National Institutes of Health under Ruth L. Kirsch stein National Research Service Award T32 AR007505 from the NIH NIAMS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33:832–9. doi: 10.1002/jor.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11:909–17. doi: 10.3928/0147-7447-19880601-11. [DOI] [PubMed] [Google Scholar]
- 3.Harris SB, Harris D, Foster AJ, Elliot D. The aetiology of acute rupture of flexor tendon repairs in zones 1 and 2 of the fingers during early mobilization. J Hand Surg Br. 1999;24:275–80. doi: 10.1054/jhsb.1998.0212. [DOI] [PubMed] [Google Scholar]
- 4.Woo SL, Gelberman RH, Cobb NG, Amiel D, Lothringer K, Akeson WH. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop Scand. 1981;52:615–22. doi: 10.3109/17453678108992156. [DOI] [PubMed] [Google Scholar]
- 5.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–94. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 6.Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg Am. 2005;30:441–7. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Costa MA, Wu C, Pham BV, Chong AK, Pham HM, Chang J. Tissue engineering of flexor tendons: optimization of tenocyte proliferation using growth factor supplementation. Tissue Eng. 2006;12:1937–43. doi: 10.1089/ten.2006.12.1937. [DOI] [PubMed] [Google Scholar]
- 8.James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33:102–12. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Ramanath HS, Wang DA. Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol. 2008;26:201–9. doi: 10.1016/j.tibtech.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa Y, Abrahamsson SO. Dose-related cellular effects of platelet-derived growth factor-BB differ in various types of rabbit tendons in vitro. Acta Orthop Scand. 2001;72:287–92. doi: 10.1080/00016470152846646. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamsson SO, Lundborg G, Lohmander LS. Recombinant human insulin-like growth factor-I stimulates in vitro matrix synthesis and cell proliferation in rabbit flexor tendon. J Orthop Res. 1991;9:495–502. doi: 10.1002/jor.1100090405. [DOI] [PubMed] [Google Scholar]
- 12.Zhang F, Liu H, Stile F, Lei MP, Pang Y, Oswald TM, et al. Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plastic and Reconstructive Surgery. 2003;112:1613–9. doi: 10.1097/01.PRS.0000086772.72535.A4. [DOI] [PubMed] [Google Scholar]
- 13.Dines JS, Cross MB, Dines D, Pantazopoulos C, Kim HJ, Razzano P, et al. In vitro analysis of an rhGDF-5 suture coating process and the effects of rhGDF-5 on rat tendon fibroblasts. Growth Factors. 2011;29:1–7. doi: 10.3109/08977194.2010.526605. [DOI] [PubMed] [Google Scholar]
- 14.Henn RF, Kuo CE, Kessler MW, Razzano P, Grande DP, Wolfe SW. Augmentation of Zone II Flexor Tendon Repair Using Growth Differentiation Factor 5 in a Rabbit Model. Journal of Hand Surgery-American Volume. 2010;35A:1825–32. doi: 10.1016/j.jhsa.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Dines JS, Weber L, Razzano P, Prajapati R, Timmer M, Bowman S, et al. The effect of growth differentiation factor-5-coated sutures on tendon repair in a rat model. J Shoulder Elbow Surg. 2007;16:S215–21. doi: 10.1016/j.jse.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Thomopoulos S, Kim HM, Das R, Silva MJ, Sakiyama-Elbert S, Amiel D, et al. The effects of exogenous basic fibroblast growth factor on intrasynovial flexor tendon healing in a canine model. J Bone Joint Surg Am. 92:2285–93. doi: 10.2106/JBJS.I.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomopoulos S, Zaegel M, Das R, Harwood FL, Silva MJ, Amiel D, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25:1358–68. doi: 10.1002/jor.20444. [DOI] [PubMed] [Google Scholar]
- 18.Younesi M, Islam A, Kishore V, Anderson JM, Akkus O. Tenogenic Induction of Human MSCs by Anisotropically Aligned Collagen Biotextiles. Adv Funct Mater. 2014;24:5762–70. doi: 10.1002/adfm.201400828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younesi M, Islam A, Kishore V, Panit S, Akkus O. Fabrication of compositionally and topographically complex robust tissue forms by 3D-electrochemical compaction of collagen. Biofabrication. 2015;7:035001. doi: 10.1088/1758-5090/7/3/035001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Islam A, Chapin K, Younesi M, Akkus O. Computer aided biomanufacturing of mechanically robust pure collagen meshes with controlled macroporosity. Biofabrication. 2015;7:035005. doi: 10.1088/1758-5090/7/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uquillas JA, Akkus O. Modeling the electromobility of type-I collagen molecules in the electrochemical fabrication of dense and aligned tissue constructs. Ann Biomed Eng. 2012;40:1641–53. doi: 10.1007/s10439-012-0528-1. [DOI] [PubMed] [Google Scholar]
- 22.Alfredo Uquillas J, Kishore V, Akkus O. Genipin crosslinking elevates the strength of electrochemically aligned collagen to the level of tendons. J Mech Behav Biomed Mater. 2012;15:176–89. doi: 10.1016/j.jmbbm.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Younesi M, Donmez BO, Islam A, Akkus O. Heparinized collagen sutures for sustained delivery of PDGF-BB: Delivery profile and effects on tendon-derived cells In-Vitro. Acta Biomater. 2016;41:100–9. doi: 10.1016/j.actbio.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheffler SU, Scherler J, Pruss A, von Versen R, Weiler A. Biomechanical comparison of human bone-patellar tendon-bone grafts after sterilization with peracetic acid ethanol. Cell Tissue Bank. 2005;6:109–15. doi: 10.1007/s10561-004-6403-z. [DOI] [PubMed] [Google Scholar]
- 25.Islam A, Bohl MS, Tsai AG, Younesi M, Gillespie R, Akkus O. Biomechanical evaluation of a novel suturing scheme for grafting load-bearing collagen scaffolds for rotator cuff repair. Clinical Biomechanics. 2015;30:669–75. doi: 10.1016/j.clinbiomech.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Percival KR, Radi ZA. A modified Verhoeff-van Gieson elastin histochemical stain to enable pulmonary arterial hypertension model characterization. European Journal of Histochemistry. 2016;60:70–4. doi: 10.4081/ejh.2016.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jilani SM, Murphy TJ, Thai SNM, Eichmann A, Alva JA, Iruela-Arispe ML. Selective binding of lectins to embryonic chicken vasculature. Journal of Histochemistry & Cytochemistry. 2003;51:597–604. doi: 10.1177/002215540305100505. [DOI] [PubMed] [Google Scholar]
- 28.Nag S. Ultrastructural-Localization of Lectin Receptors on Cerebral Endothelium. Acta Neuropathologica. 1985;66:105–10. doi: 10.1007/BF00688684. [DOI] [PubMed] [Google Scholar]
- 29.Robertson RT, Levine ST, Haynes SM, Gutierrez P, Baratta JL, Tan ZQ, et al. Use of labeled tomato lectin for imaging vasculature structures. Histochemistry and Cell Biology. 2015;143:225–34. doi: 10.1007/s00418-014-1301-3. [DOI] [PubMed] [Google Scholar]
- 30.Khan U, Kakar S, Akali A, Bentley G, McGrouther DA. Modulation of the formation of adhesions during the healing of injured tendons. J Bone Joint Surg Br. 2000;82:1054–8. doi: 10.1302/0301-620x.82b7.9892. [DOI] [PubMed] [Google Scholar]
- 31.Tang JB. Clinical outcomes associated with flexor tendon repair. Hand Clin. 2005;21:199–210. doi: 10.1016/j.hcl.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 32.May EJ, Silfverskiold KL. Rate of recovery after flexor tendon repair in zone II. A prospective longitudinal study of 145 digits. Scand J Plast Reconstr Surg Hand Surg. 1993;27:89–94. doi: 10.3109/02844319309079789. [DOI] [PubMed] [Google Scholar]
- 33.Thomopoulos S, Das R, Silva MJ, Sakiyama-Elbert S, Harwood FL, Zampiakis E, et al. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res. 2009;27:1209–15. doi: 10.1002/jor.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, Das R, Silva MJ. The early effects of sustained platelet-derived growth factor administration on the functional and structural properties of repaired intrasynovial flexor tendons: an in vivo biomechanic study at 3 weeks in canines. J Hand Surg Am. 2007;32:373–9. doi: 10.1016/j.jhsa.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Letson AK, Dahners LE. The effect of combinations of growth factors on ligament healing. Clin Orthop Relat Res. 1994:207–12. [PubMed] [Google Scholar]
- 36.Hildebrand KA, Woo SL, Smith DW, Allen CR, Deie M, Taylor BJ, et al. The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study. Am J Sports Med. 1998;26:549–54. doi: 10.1177/03635465980260041401. [DOI] [PubMed] [Google Scholar]
- 37.Risau W, Drexler H, Mironov V, Smits A, Siegbahn A, Funa K, et al. Platelet-derived growth factor is angiogenic in vivo. Growth Factors. 1992;7:261–6. doi: 10.3109/08977199209046408. [DOI] [PubMed] [Google Scholar]
- 38.Saik JE, Gould DJ, Watkins EM, Dickinson ME, West JL. Covalently immobilized platelet-derived growth factor-BB promotes angiogenesis in biomimetic poly(ethylene glycol) hydrogels. Acta Biomater. 2011;7:133–43. doi: 10.1016/j.actbio.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994;125:917–28. doi: 10.1083/jcb.125.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–13. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 41.Khachigian LM, Resnick N, Gimbrone MA, Jr, Collins T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest. 1995;96:1169–75. doi: 10.1172/JCI118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 43.Bar RS, Boes M, Booth BA, Dake BL, Henley S, Hart MN. The effects of platelet-derived growth factor in cultured microvessel endothelial cells. Endocrinology. 1989;124:1841–8. doi: 10.1210/endo-124-4-1841. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Cao R, Zhang Y, Jia T, Cao Y, Wahlberg E. Differential roles of PDGFR-alpha and PDGFR-beta in angiogenesis and vessel stability. FASEB J. 2009;23:153–63. doi: 10.1096/fj.08-113860. [DOI] [PubMed] [Google Scholar]


