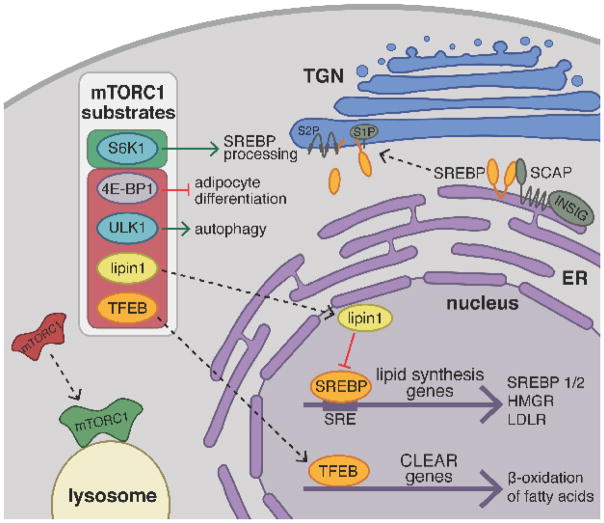
Fig. 3. mTORC1 activation promotes lipid biogenesis and suppresses lipid catabolism.
In the presence of nutrients, mTORC1 translocates from the cytoplasm to the surface of the lysosome where it becomes activated and phosphorylates downstream substrates thereby activating (green box) or inhibiting them (red box). S6-kinase 1 (S6K1) is an mTORC1 substrate that promotes proteolytic processing of the transcription factors SREBP1/2. Inactive, full-length SREBP is retained at the ER membrane by its association with SCAP and INSIG in the presence of cholesterol. Under low cholesterol conditions in the ER, SCAP undergoes a conformational change releasing SREBP and promoting its COPII vesicle mediated trafficking to the Golgi. At the Golgi, two proteases, S1P and S2P, cleave SREBP to release the soluble, N-terminal fragment, which translocates to the nucleus and binds to sterol response elements (SRE) upstream of promoters for genes involved in sterol uptake and de novo lipid biosynthesis. Furthermore, mTORC1 phosphorylation blocks the nuclear entry of lipin1, a phosphatidic acid phosphatase that acts as an inhibitor of nuclear SREBP activity. Similarly, phosphorylation of TFEB prevents its translocation to the nucleus, repressing transcriptional activation of CLEAR genes involved in β-oxidation of fatty acids, lipid catabolism, and lysosomal biogenesis. mTORC1 is thought to promote adipocyte differentiation by releasing a translational block mediated by 4E-BP1. Finally, mTORC1 inhibits autophagic degradation of lipid droplets through phosphorylation of ULK1 kinase.