Abstract
Objective
To devise a minimally invasive, less morbid yet effective alternative technique for basilic vein transposition (BVT) in the arm/forearm and to compare perioperative outcomes with the conventional technique.
Patients and methods
Patients undergoing BVT in the last two years (June 2013 to June 2015) were included in the study and the results were analysed. All patients were preoperatively evaluated using colour Doppler ultrasonography performed by the operating surgeon himself. For minimally invasive BVT, two or three small 1–2 cm incisions were made to completely mobilise the basilic vein, transposed in an anterolateral arm/forearm tunnel, and then anastomosed to the brachial or radial artery in the forearm and arm, respectively. The incision in the conventional technique was along the full length of the basilic vein, with the rest of the procedure remaining the same. Complications, pain, analgesic use, maturation and primary patency rates were compared between the techniques.
Results
In all, 30 patients underwent minimally invasive BVT and 34 patients underwent conventional BVT, with mean age of 52 and 55 years, respectively. The complications of wound haematoma (one vs four) and wound infection/dehiscence (two vs six) were less common in the minimally invasive BVT group compared to the conventional group. The analgesic requirement and visual analogue scale pain score was significantly less in the minimally invasive BVT group. All other variables assessed, such as maturation and primary patency rate at 1 year, were not significantly different between the groups.
Conclusion
Minimally invasive dissection of the basilic vein for vascular access transposition is a safe, reliable procedure with patency and functional outcomes comparable with those of conventional BVT.
Abbreviations: AVF, arteriovenous fistula; BVT, basilic vein transposition; KDOQI, Kidney Disease Outcome Quality Initiative; s.c., subcutaneous; US, ultrasonography
Keywords: Basilic vein, Brachial artery, Minimally invasive, Fistula, Transposition
Introduction
For patients with end-stage renal disease, the Kidney Disease Outcome Quality Initiative (KDOQI) guidelines recommend autogenous Brescia-Cimino (radiocephalic) or brachiocephalic fistula as the preferred type of vascular access [1]. But in patients where the cephalic vein is poorly developed, not patent, thrombosed, or if such access fails, the fistula of choice is basilic vein transposition (BVT) before switching onto complicated vascular graft surgery, as is suggested by KDOQI guidelines in 2006 [2]. The first description of BVT was in 1976 and since then it has been progressively accepted as a feasible option for failed cases of vascular access [3]. Using a videoendoscopic technique to dissect the basilic vein encouraged us to devise a minimally invasive procedure to perform BVT, with results comparable to the conventional technique but with less complications as a result of limiting the dissection. We previously described a minimally invasive BVT technique in 2010 [4]. Since then the technique has been modified and now has become more refined maintaining the functional outcome while further decreasing the related complications. The obvious disadvantages of the minimally invasive procedure are the longer operative time and that it is technically more demanding than the conventional technique. In the present study, we present a more refined and standardised minimally invasive technique and compare this new method with the traditional technique to perform BVT for perioperative outcomes.
Patients and methods
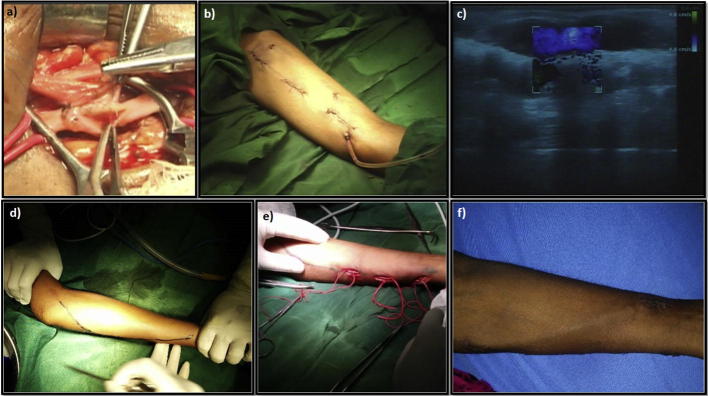
All patients in the last two years (from June 2013 to June 2015) undergoing BVT fistula, after preoperative evaluation by coloured Doppler ultrasonography (US), were included in the study. All these patients had either failed radiocephalic, in the case of forearm transposition, failed radiocephalic and brachiocephalic fistula in case of BVT. Also, if on Doppler US there was a poorly developed/absent cephalic vein then BVT was planned. Coloured Doppler US in our institute is routinely performed by the trained surgeon himself. Based on our own Doppler US experience and extensive review of the literature, we have devised a minimum diameter/maximal depth of brachial artery and basilic vein for optimal results (Fig.1a and b) [3], [5], as this helps immensely in planning and prognostication. On table Doppler US is repeated to map the artery and vein, which minimises the need for extensive dissection (Fig.1c and d).
Fig. 1.
(a, b) Minimum diameter/maximal depth of brachial artery and basilic vein for optimal results. (c, d) Intraoperative coloured Doppler US to map artery and vein.
Technique
The procedure is performed either with a brachial (upper limb) block with local anaesthesia or under general anaesthesia as determined by the anaesthesiologist.
Minimally invasive BVT in arm
After appropriate mapping of the artery and vein, a 2-cm incision is made in the medial aspect of the arm at the level of the cubital fossa along the length of the mapped vein. The basilic vein is identified and dissected. The superficial investing fascia layer is incised longitudinally along the length of the vein to achieve a maximum working space with a minimum length of incision. The proximal, distil end of the vein is ‘slinged’ and lifted up (Fig.2a). The vein is freed from the perivenous tissue using cautery (monopolar) (Fig.2a). Larger tributaries of the vein are tied, while smaller ones are coagulated (bipolar cautery) (Fig.2b). Once it is felt that further dissection is not possible from this incision, a small artery forceps is inserted under vision along the length of vein underneath the skin to mark the site of the start of the next incision (skip incisions) (Fig.2c). The vein underlying the undivided skin is also freed in a similar manner. Hook/right-angle retractors (Fig.2d and e) enable proper skin lifting thus facilitating in freeing the vein present underneath the skin tunnel. Dissection of the basilic vein proceeds proximally up to the deltopectoral groove in a similar fashion (Fig.2f), until the whole length of the vein becomes free. The basilic vein is then divided near its confluence in the cubital fossa and delivered out from the most proximal incision (Fig.3a and b). At this point, an infant feeding tube (5–7 F) is inserted into the vein and flushed with saline and back flow checked. This is done to check the patency of the vein lumen (Fig.3c). A bulldog clamp is applied at the proximal end of the dissected vein (Fig.3d) and saline is again flushed through the infant feeding tube into the vein lumen. This results in hydrodistention of the vein, allowing for the identification of any leaks (Fig.3e), i.e. any small unsecured tributaries or to unrecognised injury to the vein during dissection. If a leak is present it needs to be sealed either in the form of an underrun using 6-0 polypropylene suture (Prolene®; Ethicon Endo-Surgery, Cincinnati, OH, USA) for smaller leaks or using a 4-0 silk tie (Fig.3e, inset). The exteriorised vein is then prepared for transpositioning along the flexor/anterior aspect of the arm in a subcutaneous (s.c.) tunnel, such that there is no acute angulation along the whole length of vein and specifically at the proximal and distil ends (Fig.3f). This can be achieved either by using long artery forceps or by using a perforating catheter. A small incision is made proximally in the upper arm (Fig.4a) and distally near the cubital fossa (Fig.4b), the s.c. tunnel is made using either the artery forceps or perforating catheter. The distil end of the vein to be transposed is attached to the proximal end of the perforator (Fig.4e, insert), gently channelled along the s.c. plane formed previously, and finally delivered at the cubital fossa incision (Fig.4c–e). The lie of the vein is checked by re-introduction of the feeding tube into the vein and allowing it pass to ∼25 cm, also saline is flushed and thrill palpated, and once this is done we look for any evidence of torsion at the level of the axilla, back flow may also help us decide about the lie. Back flow was again checked here (Fig.4f), which indirectly confirms the correct lie of the vein in the tunnel. The end (transposed basilic vein)-to-side (brachial artery) single-layer anastomosis is made using 6-0 polypropylene suture under loupes magnification (×2.5–4) with the aid of a head light (Fig.5a). After completion of the anastomosis (Fig.5b), the fistula is examined for a thrill and bruit, which confirms good flow across the anastomosis. An on table Doppler US is repeated to ascertain the flow of blood through the transposed vein (Fig.5c) and a s.c. suction drain is placed to prevent seroma formation avoiding a tamponade effect on the arteriovenous fistula (AVF). A single dose of low-molecular-weight heparin is given and handballing exercises started 4 h postoperatively. The suction drain is removed after 48 h or when draining <20 mL/24 h, whichever is earlier. All AVF patients are administered a single aspirin tablet (Ecosprin 150 mg) from the first postoperative day.
Fig. 2.
(a) Proximal, distil end of vein ‘slinged’, lifted up and freed from perivenous tissue with help of cautery (monopolar). (b) Larger tributaries tied while smaller ones coagulated (bipolar). (c) Technique to mark skip incision. (d, e) Technique to use hook/right-angle retractors for skin lifting to dissect underneath skin tunnel. (f) Fully freed basilic vein dissected up to the axilla.
Fig. 3.
(a, b) Basilic vein gently pulled out from the proximal incision and fully exteriorised. (c) Saline flush test and backflow checked. (d) Vein hydrodistention (with heparinised saline) increases vein diameter and allows for the identification of any leaks (saline leak test). (3e) Saline leak test if positive, larger leaks tied using silk 4-0, smaller leaks – underrun using 6-0 polypropylene suture. (3f) Exteriorised vein planned for transposition on anterior surface of arm avoiding any acute angulation/kink at proximal (lower inset) or distil end (upper inset).
Fig. 4.
(a, b) Small incision is made proximally in upper arm and distally near cubital fossa as planned (to avoid acute angulation), a small s.c. tunnel is made using either artery forceps or perforating catheter. (c–e) The distil end of the basilic vein is tied over the proximal end of the perforating catheter (e, inset), tunnelled along the s.c. plane and finally delivering in the cubital fossa incision. (f) Backflow is again checked to confirm correct lie of vein in the s.c. tunnel.
Fig. 5.
(a) End (transposed basilic vein)-to-side (brachial artery) anastomosis is made using 6-0 polypropylene suture under loupes magnification. (b) Gross appearance after completion of minimally invasive BVT in arm. (c) Postoperative Doppler US showing good flow through the vein. (d) Intraoperative coloured Doppler US to map artery and vein in forearm. (e) Skip incisions used to dissect and mobilise basilic vein in the forearm from the wrist to elbow using same basic principles as in the arm. (f) Final appearance of the minimally invasive BVT after transposition of the vein in the forearm.
Minimally invasive BVT in forearm
The basilic vein and radial artery are mapped in a similar way (Fig.5d). Skip incisions are used to dissect and mobilise the basilic vein in the ulnar aspect of the forearm from the wrist to the elbow using the same basic principles as in the arm (Fig.5e). The mobilised basilic vein is transposed at the flexor aspect of the forearm in a s.c. tunnel. The end (basilic vein)-to-side (radial artery) single-layer anastomosis is again made using 6-0 polypropylene suture. The rest of the procedure is the same as for the minimally invasive BVT in arm.
Conventional BVT
Conventional BVT involves a larger incision along the whole of length of the basilic vein on the medial aspect of the arm/forearm (Fig.6a and b). Then the vein is transposed anterolaterally, as in the minimally invasive BVT, using small incisions and creating a s.c. tunnel. Anastomosis of the vein and artery is made in the standard fashion as described previously.
Fig. 6.
(a) Extensive dissection is needed in the conventional BVT technique. (b) Gross appearance after completion of conventional BVT in the arm.
Complications, pain, analgesic use, maturation and primary patency rates were compared between the minimally invasive and conventional BVT groups. Pain was assessed using a visual analogue scale (VAS; 0–10).
Comparisons between the groups were made by means of the chi-square test and Student’s t-test using the Statistical Package for the Social Sciences software (SPSS® version 15.0; SPSS Inc., Chicago, IL, USA).
Results
In all, 30 patients underwent the minimally invasive BVT and 34 underwent conventional BVT, with a mean age of 52 and 55 years, respectively (Table 1). The mean (SD) postoperative VAS score at 24 and 48 h were 2.49 (0.95) and 2.45 (1.15) in minimally invasive group and 3.16 (1.55) and 3.02 (1.09) in the conventional technique group. Also, the mean (SD) total postoperative analgesic requirement in the minimally invasive and conventional BVT groups was 315 (72) and 350 (63) mg of diclofenac sodium, respectively. Maturation and patency rates at 1 year were comparable in both groups (Table 2). The complications of wound haematoma (one vs four) and wound infection/dehiscence (two vs six) were less in the minimally invasive BVT group vs the conventional technique group (Table 3).
Table 1.
The patients’ perioperative characteristics.
| Characteristic | Minimally invasive BVT | Conventional BVT | P |
|---|---|---|---|
| Number of patients | 30 | 34 | |
| Number of arm BVT | 24 | 32 | |
| Number of forearm BVT | 6 | 2 | |
| Mean (SD): | |||
| Age, years | 52.4 (14) | 55.5 (15) | 0.398 |
| Body mass index, kg/m2 | 26.8 (6) | 24.4 (5) | 0.085 |
| Co-morbidity, n | NA | ||
| Diabetes mellitus | 14 | 17 | |
| Hypertension | 20 | 18 | |
| Ischaemic heart disease/vascular | 5 | 6 | |
| Anaesthesia type, n | NA | ||
| General | 12 | 18 | |
| Block | 18 | 16 | |
| History of previous access, n | 24 | 23 | NA |
| Mean (SD): | |||
| Operative time, min | 236 (47) | 218 (33) | 0.078 |
| Analgesic requirement (diclofenac sodium injection), mg | 315 (72) | 350 (63) | 0.042 |
| 24-h VAS | 2.49 (0.95) | 3.16 (1.55) | 0.044 |
| 48-h VAS | 2.45 (1.15) | 3.02 (1.09) | 0.046 |
NA, not applicable.
Table 2.
Functional results of BVT fistulae in relation to surgical technique.
| Variable | Minimally invasive BVT (n = 24 arm, 6 forearm) | Conventional BVT (n = 32 arm, 2 forearm) |
|---|---|---|
| % BVT fistula maturation at: | ||
| 6 weeks | 73.4 | 70 |
| 12 weeks | 82 | 83.6 |
| Patency rate at 1 year,% | 69 | 73 |
Table 3.
Distribution of postoperative complications of BVT fistulae in relation to surgical technique.
| Complication, n (%) | Minimally invasive BVT (n = 24 arm, 6 forearm) | Conventional BVT V(n = 32 arm, 2 forearm) | P |
|---|---|---|---|
| Wound haematoma | 1 (3.33) | 4 (11.8) | 0.43 |
| Wound dehiscence or infection | 2 (6.67) | 6 (17.6) | 0.34 |
| Steal syndrome | 1 (3.33) | 1 (2.94) | 0.52 |
| Failure to mature | 3 (10) | 4 (11.8) | 0.86 |
| Thrombosis | |||
| Acute | 1 (3.33) | 1 (2.94) | 0.53 |
| Chronic | 2 (6.67) | 3 (8.82) | 0.89 |
| Venous hypertension | 2 (6.67) | 5 (14.7) | 0.39 |
Discussion
In the present study we have demonstrated a novel and more refined technique of minimally invasive BVT and shown its superiority in terms of morbidity and postoperative complications compared with the conventional BVT technique. For patients who have exhausted other autologous options for fistula formation, the basilic vein is a crucial and cost-effective option when compared to a prosthetic graft [6].
Preoperative colour Doppler US assessment along with clinical examination forms the cornerstone of the preoperative evaluation. We recommend that the operating surgeon is appropriately trained to perform colour Doppler US, as it helps immensely in surgery planning and prognostication. The minimum diameter of the basilic vein at the cubital fossa and wrist that we recommend is 3 mm and 2.5 mm, respectively. This is based on our experience, as well as on review of the literature [3], [5]. To improve the result of BVT we have incorporated various technical modifications such as: on table mapping of the artery and vein to minimise dissection, making the anastomosis under loupes magnification (×2.5–4) with the aid of a head light, superficialisation as well as transposition of the basilic vein, preventing angulation while doing anterolateral transposition, hydrodistending the vein to increase its lumen diameter and to identify any leaks, and the use of a suction drain to prevent seroma formation.
Although minimally invasive BVT takes longer than the conventional technique, it was not statistically significantly different. More time is required in for minimally invasive BVT to dissect underneath the skip incisions; however, this is to some extent counterbalanced by the lesser time needed for small incision closure. The mean VAS and total analgesic requirement was significantly less in the minimally invasive group in first 48 h, probably because minimally invasive BVT involves lesser dissection, smaller incisions and hence, less postoperative pain that requires lesser analgesia.
Immediate postoperative limb oedema, as well as the number of wound complications, such as wound infection, wound dehiscence, and wound haematoma were less in the minimally invasive technique as compared to the conventional technique, although not statistically significantly so. This is probably attributable to the minimum dissection, lesser manipulation, and skip incisions (in part attributed to preoperative on table colour Doppler US), which further diminishes the chances of lymphatic disruption, wound haematoma and wound infection leading to lesser wound dehiscence and better earlier wound healing. Less limb oedema leads to less compartmental pressure and theoretically less chance of outflow obstruction/thrombosis. Venous hypertension was also more commonly seen in the conventional group. This could be due to haematoma that can aggravate venous hypertension by compressing outflow of blood through the venous channel and vice versa. The incidence of venous hypertension is quite variable ranging from 3.6% to 25% due to difference in reporting criterion by different authors [7], [8].
Other complications, such as steal syndrome, thrombosis, and maturation failure, were comparable in both groups. This may be due to the fact that these variables depend largely on individual patient characteristics (if the anastomotic technique remains the same) rather than whether the procedure is minimally invasive or conventional. Both patients with steal syndrome (one in each group) required fistula closure, which has a reported incidence of up to 6.5% [9]. Both patients (one in each group) who had acute thrombosis reported early and were explored. The thrombus was extracted using a Fogarty catheter. Postoperatively to prevent further thrombosis, both were started on a heparin infusion for the first 48 h.
The incidence of maturation failure was comparable in the groups (10–12%), which is comparable to the published incidence that varies from 3% to 35% based on different techniques, selection criterion, Doppler US practices, and institute expertise [7], [10], [11]. The time to maturation in our present study is also consistent with published reports, which vary from centre to centre viz. 1.5–4.5 months [7], [10], [12]. One patient in each group required balloon dilatation of a stenosed anastomosis.
The main limitation of our present study is that it is a retrospective comparison between two techniques with a lack of long-term results. Secondly, our anaesthesia technique was also not generalised, which might have a bearing on the results [13]. Further randomised trials with a strict preoperative evaluation protocol will be required to validate the optimum size of the vein and artery, as well as the superiority of minimally invasive BVT over the conventional technique in terms of lesser morbidity.
Conclusion
In conclusion, minimally invasive dissection of the basilic vein for vascular access transposition is a safe, reproducible procedure with less overall morbidity and wound-related complications, without compromising patency and functional outcomes, as compared to conventional BVT.
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Source of Funding
None.
Point of Technique
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.National Kidney Foundation KDOQI Clinical Practice Guidelines for Vascular Access, 2000. Am J Kidney Dis. 2001;37(Suppl. 1):S137–S181. doi: 10.1016/s0272-6386(01)70007-8. [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation KDOQI clinical practice guidelines and clinical practice recommendations for 2006 updates: hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis. 2006;48(Suppl. 1):S1–S322. [Google Scholar]
- 3.Sidawy A.N., Gray R., Besarab A., Henry M., Ascher E., Silva M., Jr Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg. 2002;35:603–610. doi: 10.1067/mva.2002.122025. [DOI] [PubMed] [Google Scholar]
- 4.Veeramani M., Vyas J., Sabnis R., Desai M. Small incision basilic vein transposition technique: a good alternative to standard method. Indian J Urol. 2010;26:145–147. doi: 10.4103/0970-1591.60466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown P.W. Preoperative radiological assessment for vascular access. Eur J Vasc Endovasc Surg. 2006;31:64–69. doi: 10.1016/j.ejvs.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Lazarides M.K., Georgiadis G., Papasideris C., Trellopoulos G., Tzilalis V.D. Transposed brachial-basilic arteriovenous fistulas versus prosthetic upper limb grafts: a meta-analysis. Eur J Vasc Endovasc Surg. 2008;36:597–601. doi: 10.1016/j.ejvs.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Murphy G.J., White S.A., Knight A.J., Doughman T., Nicholson M.L. Long-term results of arteriovenous fistulas using transposed autologous basilic vein. Br J Surg. 2000;87:819–823. doi: 10.1046/j.1365-2168.2000.01435.x. [DOI] [PubMed] [Google Scholar]
- 8.Hossny A. Brachiobasilic arteriovenous fistula: different surgical techniques and their effects on fistula patency and dialysis related complications. J Vasc Surg. 2003;37:821–826. doi: 10.1067/mva.2003.181. [DOI] [PubMed] [Google Scholar]
- 9.Butterworth P.C., Doughman T.M., Wheatley T.J., Nicholson M.L. Arteriovenous fistula using transposed basilic vein. Br J Surg. 1998;85:653–654. doi: 10.1046/j.1365-2168.1998.00654.x. [DOI] [PubMed] [Google Scholar]
- 10.Rao R.K., Azin G.D., Hood D.B., Rowe V.L., Kohl R.D., Katz S.G. Basilic vein transposition fistula: a good option for maintaining hemodialysis access site options? J Vasc Surg. 2004;39:1043–1047. doi: 10.1016/j.jvs.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Keuter X.H., van der Sande F.M., Kessels A.G., de Haan M.W., Hoeks A.P., Tordoir J.H. Excellent performance of one-stage brachial-basilic arteriovenous fistula. Nephrol Dial Transplant. 2005;20:2168–2171. doi: 10.1093/ndt/gfh997. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald J.T., Schanzer A., Chin A.I., McVicar J.P., Perez R.V., Troppmann C. Outcomes of upper arm arteriovenous fistulas for maintenance hemodialysis access. Arch Surg. 2004;139:201–208. doi: 10.1001/archsurg.139.2.201. [DOI] [PubMed] [Google Scholar]
- 13.Hingorani A.P., Ascher E., Gupta P., Alam S., Marks N., Schutzer R.W. Regional anesthesia: preferred technique for venodilatation in the creation of upper extremity arteriovenous fistulae. Vascular. 2006;14:23–26. doi: 10.2310/6670.2006.00006. [DOI] [PubMed] [Google Scholar]