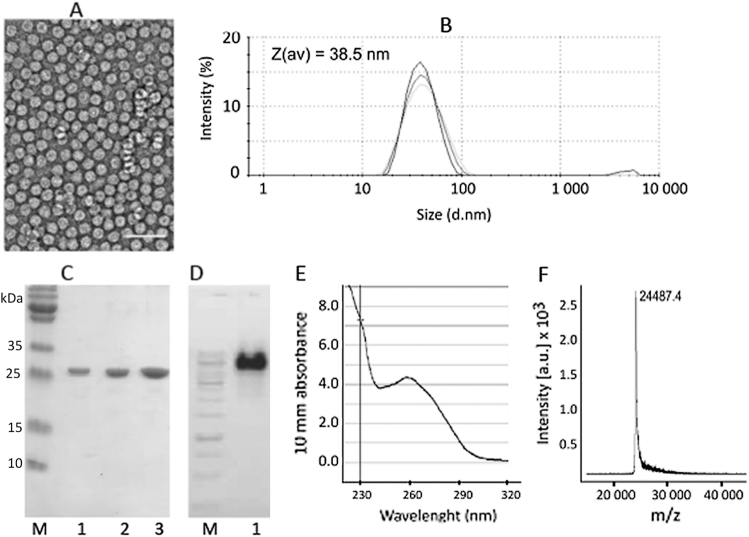
Fig. 1.
Properties of CMVTT virus-like particles. a Electron microscopy image of purified VLPs incorporating the universal T-cell epitope (Gln Tyr Ile Lys Ala Asn Ser Lys Phe Ile Gly Ile Thr Glu) derived from Tetanus toxin. VLP sample (1.5 mg/ml) was adsorbed on carbon formvar-coated copper grids and were negatively stained with 1% uranyl acetate aqueous solution. The grids were examined using a JEM-100C electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 80 kV. White bar corresponds to 100 nm. Particle size was found between 26 and 28 nm. b Dynamic light scattering (DLS) analysis of CMVTT. Sample VLP solution (1 mg/ml) was analyzed on a Zetasizer Nano ZS instrument (Malvern Instruments Ltd, UK). The results of three measurements were analyzed by DTS software (Malvern, version 6.32). The average hydrodynamic diameter (Z(av)) of particles was found 38.5 nm. c SDS-PAGE analysis of CMVTT VLPs. Increasing amounts of VLPs were loaded on the gel (lane 1–0.6 µg, lane 2–1.2 µg and lane 3–2.4 µg). M—protein size marker (Page Ruler Plus, Thermo Scientific). Samples were derived from the same experiment and were processed in parallel. d Agarose gel analysis of CMVTT. Lane 1 – CMV VLPs (4 µl, 1.5 mg/ml) were mixed with DNA Loading buffer (lane 1) and analyzed in 0.8% agarose /TBE buffer. M – DNA size marker (Gene Ruler, 1 kb, Thermo Scientific). Samples were derived from the same experiment and were processed in parallel. e UV spectroscopy. The UV spectrum of VLP solution (1 mg/ml) was recorded using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, USA). The VLP sample absorbs strongly at 260 nm which is typical for viruses and VLPs. f For mass spectrometric (MS) analysis CMVTT VLPs (1 mg/ml) were diluted with a 3-hydroxypicolinic acid matrix solution and were spotted onto an MTP AnchorChip 400/384TF. Matrix-assisted laser desorption/ionization (MALDI)-TOF MS analysis was carried out on an Autoflex MS (Bruker Daltonik, Germany). The protein molecular mass (MM) calibration standard II (22.3–66.5 kDa; Bruker Daltonik) was used for mass determination. Obtained spectrum suggests that the first methionine is removed during the CMV coat protein synthesis in E. coli cells and the N-terminus is replaced by the TT epitope (calculated MW 24485 Da, found m/z value 24487.3 Da)