Figure 1.
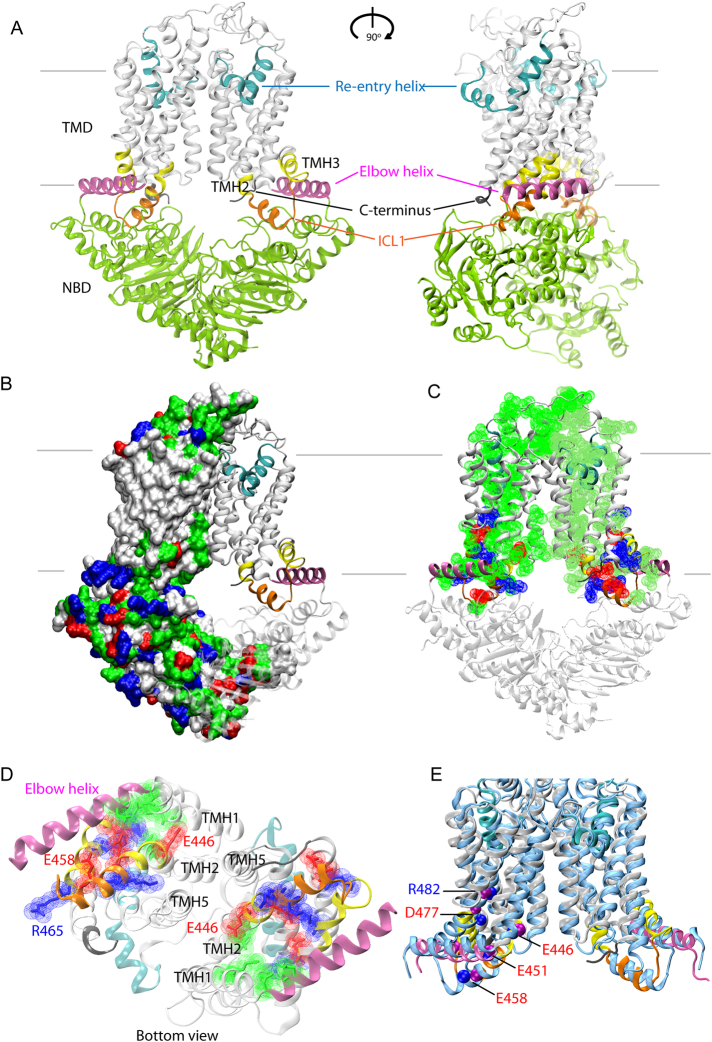
The structural model of ABCG2 reveals a novel configuration of the transmission interface. (A) Model of the homodimeric ABCG2 transporter in ATP-free state based on coordinates of ABCG5/G8 PDB ID: 5D07 in two views rotated by 90°. The conformation shows pronounced symmetry, including three distinct structural features forming the transmission interface in each monomer, including the elbow helix (pink), the first intracellular loop ICL1 (orange), and the surface of the NBD dimer (green); the extracellular re-entry helix (cyan) is protruding into the outer leaflet; cytoplasmic distal helical parts of TMH2 and TMH3 (yellow); C-terminus (black); TMHs (light grey), TMD, transmembrane domain, NBD, nucleotide binding domain. (B) Polar residues on the ABCG2 surface. The ABCG2 monomer based on ABCG5 shows residues as surface potential filling; positive charges (blue), negative charges (red), polar (green), non-polar (white). The second half is based on ABCG8 using the same color-coding of domains as above. (C) Polar residues in the TMD of ABCG2 molecule. (D) Inside-outside bottom view of ABCG2 TMDs with the NBDs removed for better overview. (E) Superimposition of TMDs in the cryo-EM structure40 of ABCG2 (PDB ID: 5Nj3) (blue) and the atomic model obtained in this study using a ribbon representation limited to the TMD regions. Color code as in panel A. Blue balls indicate residue positions, whereas purple balls indicate residues in the present atomic model. The ABCG2 cryo-EM structure PDB ID: 5Nj3 corresponds to a ABCG5 homodimer, while the present atomic model is based on the ABCG5/G8 heterodimer PDB ID: 5D07.