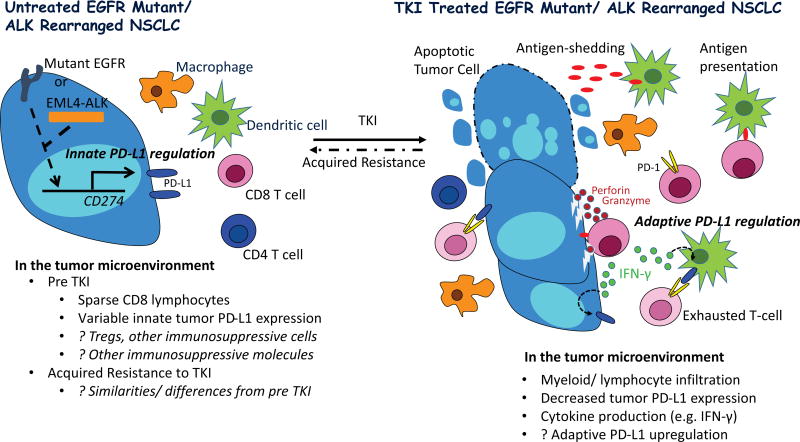
Figure 1. Proposed Tumor immunity in TKI naïve and treated EGFR and ALK Driven Lung Cancer.
Oncogenic signaling through EGFR and ALK drives tumor PD-L1 expression in preclinical models (innate PD-L1 regulation), however, PD-L1 protein expression is variable in tumor specimens from patients with EGFR and ALK driven NSCLC (2–6). Predicted lower somatic mutational burden in such tumors, relative to typical smoking associated NSCLC, may result in less immunogenic tumors, potentially explaining reports describing scarcity of tumor infiltrating lymphocytes (TILs). Preclinical studies have additionally identified tumor infiltrating T regulatory cells in EGFR mutant models, which may further promote tumor immune evasion (2).
On treatment with a highly active tyrosine kinase inhibitor (TKI), apoptosis of EGFR and ALK driven NSCLC leads to tumor influx of immune cells and cytokine production, and tumor antigen processing by dendritic cells with presentation to antigen specific T cells which can then engage tumor cells, releasing cytotoxic enzymes (perforin and granzyme) and pro-inflammatory cytokines. The latter, in particular IFN-γ, can induce PD-L1 on myeloid and tumor cells (adaptive PD-L1 regulation), resulting in T cell exhaustion with blunting of the anti-tumor immune response. Of note, preclinical studies have demonstrated PD-1 inhibitors can relieve suppression of effector T cells in EGFR and ALK driven models, resulting in tumor cell death.
At the time of acquired resistance to TKI therapy, it is unclear if the tumor immune microenvironment regains the dominant immunosuppressive features of the pre TKI state, or if additional/ different mechanisms of immunosuppression emerge.