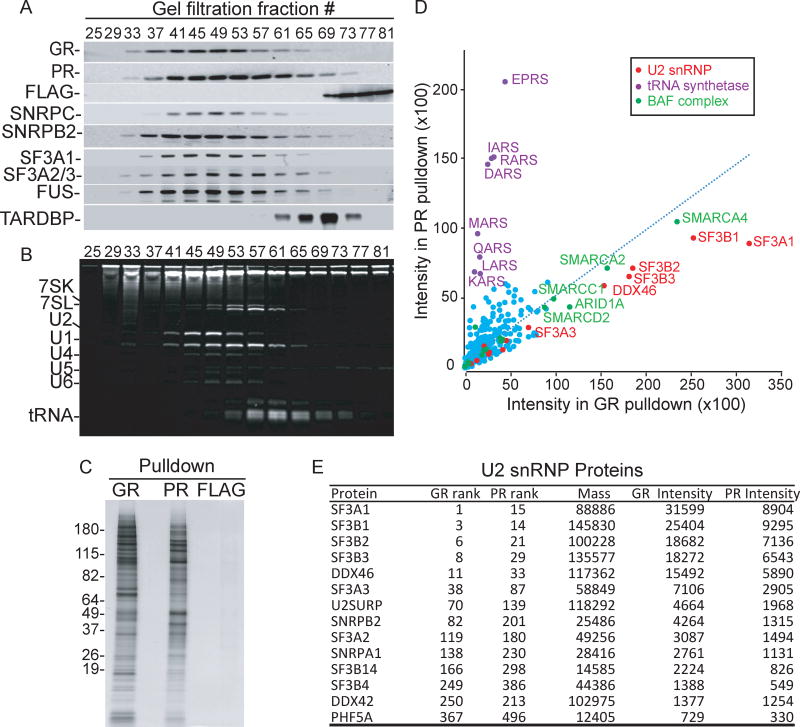
Figure 2. GR and PR associate with U2 snRNP.
(A) Nuclear extract reaction mixtures were incubated with 10 µM of either GR, PR, or FLAG peptide followed by separation on Sephacryl-S500 columns. The indicated fractions from each column were used for Western blots with an antibody against FLAG (rows GR, PR, FLAG). The GR gel filtration fractions were then used for Western blotting analysis with antibodies against the U1 snRNP component SNRPC, the U2 snRNP components SNRPB2, SF3A1, SF3A2 and SF3A3 (the latter two proteins co-migrate on the SDS gel), FUS, and TARDBP. We obtained similar results when the PR or FLAG gel filtration fractions were used for the Western blotting (data not shown). Fraction 25 is the void volume, and low molecular weight factors, such as free proteins, elute in fractions ~70–80. (B) Same as (A) except that total RNA from the GR fractions was run on an 8% denaturing gel and stained with ethidium bromide. RNA species are indicated. (C) Silver stained gel showing total proteins in GR, PR or FLAG pulldowns from gel filtration fractions 40–60. (D) Scatter plot depicting the protein intensity identified in GR or PR pulldowns. Red, purple, and green dots indicate U2 snRNP components, tRNA synthetases, and BAF complex components, respectively. (E) Table showing quantitative mass spectrometry data for the U2 snRNP components in GR or PR pulldowns. The rank of each protein in the total pulldowns sorted by mass spectrometry intensity, the calculated mass, and the mass spectrometry intensity are shown. See also Figure S2 and Tables S1–S3.